17 The Tricuspid Valve Apparatus
Introduction
The tricuspid valve (TV) plays an important role in many pathological states and its abnormality may directly or indirectly influence the morbidity and mortality in many clinical scenarios involving the right and left heart diseases. Knowing the normal morphology of the TV apparatus is important to understand and explain the underlying pathophysiological mechanisms of the TV disorders. As an example of imaging demonstration, the three-dimensional (3D) geometry of the TV annulus in normal circumstances has helped define the mechanisms for the development of functional tricuspid regurgitation (TR), a common associated condition with mitral regurgitation, and has paved ways to improve therapy. 1
In this chapter, imaging evaluation of the anatomy and function of the tricuspid apparatus with be reviewed. The embryology and cadaveric anatomy of the valve along with common pathological processes of the valve will be briefly discussed.
Formation of the Tricuspid Valve
The formation of the valvular apparatus at the atrioventricular (AV) junction is a complex process, involving the endocardial cushions, subepicardial mesenchyme, and the myocardium, but the exact contribution of the different cell types remains controversial 1 , 2 , 3 (Fig. 17‑1). The septal leaflet of the TV, the aortic leaflet of the mitral valve, and the aortomitral fibrous continuity develop from the mesenchyme of the inferior and superior AV endocardial cushions. 3 The tricuspid septal leaflet then delaminates from the muscular ventricular septum late in development. The mural leaflets including the anterior and posterior leaflets of the TV and the posterior leaflet of the mitral valve primarily originate from the mesenchymal cushions that arise laterally in the AV canal after cushion fusion. The subepicardial mesenchyme and the myocardium of the AV groove also contribute to the development of the mural leaflets. The myocardial portion is subsequently removed by apoptosis. 3 The process of myocardial cell death forms the final appearance of the leaflets and tendon apparatus. This process is called “undermining,” which delaminates the inner layers of the ventricular inlet and frees the fibrous leaflets and appears to differ somewhat for individual valve leaflets. Removal of the AV groove myocardium forms the fibrous annulus of the valve, which provides insulation between the myocardium of the atrium and that of the ventricle. 2 , 4 The epicardium will be contiguous with the insulating fibrous tissue of the fibrous annulus. This process will be completed around the 12th week of development.
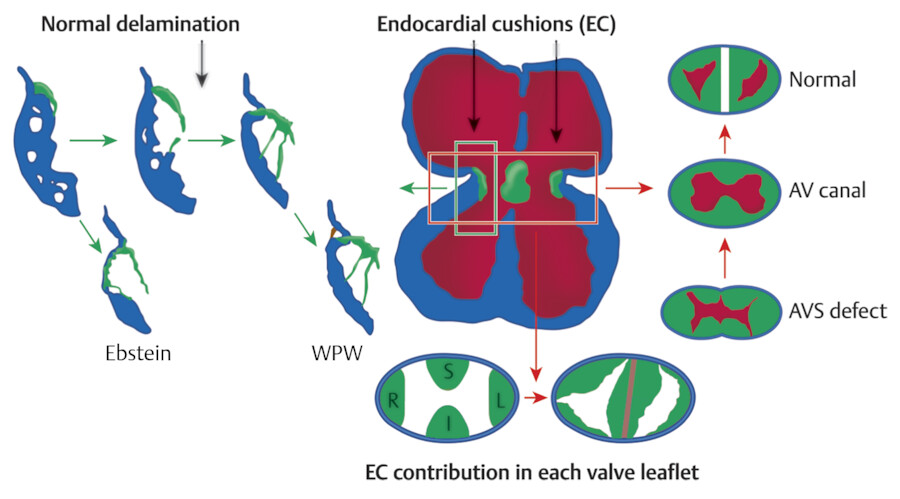
A complete failure of development of the AV cushions results in AV canal defect with variable degree of insufficiency, primum type of atrial septal defect, and perimembranous ventricular septal defect (VSD). Incomplete resorption of the myocardial tissue on the septal leaflet of the TV can result in congenital heart defects such as Ebstein’s malformation or accessory conduction pathways. 5 In Ebstein’s malformation, the attached margin of the septal leaflet and occasionally the inferior leaflet of the TV are displaced apically and “plastered” onto the right ventricular wall. 2 Myocardial AV continuities between the atrial and ventricular sides can occur. These abnormal muscular connections are known as accessory conduction pathways classically causing the arrhythmias seen in Wolff–Parkinson–White (WPW) syndrome.
Normal Tricuspid Valve
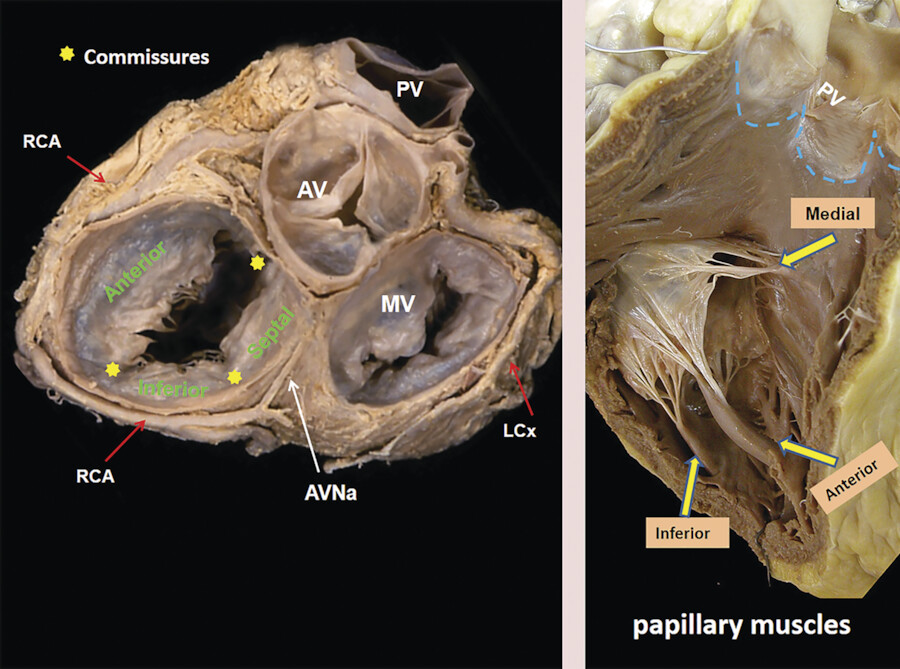
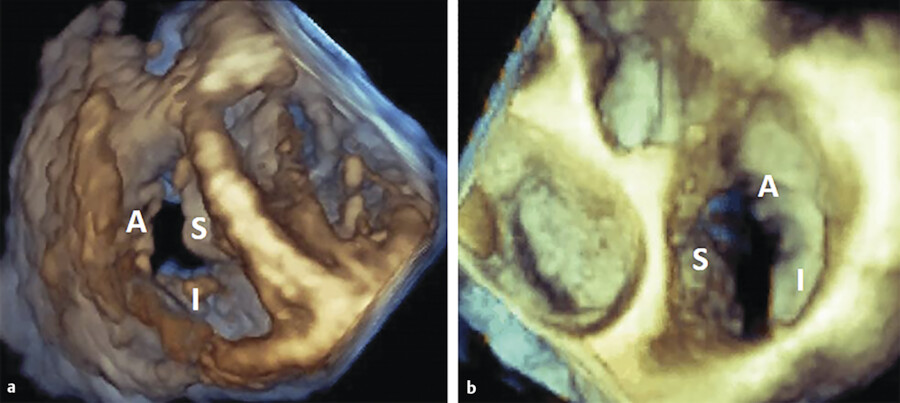
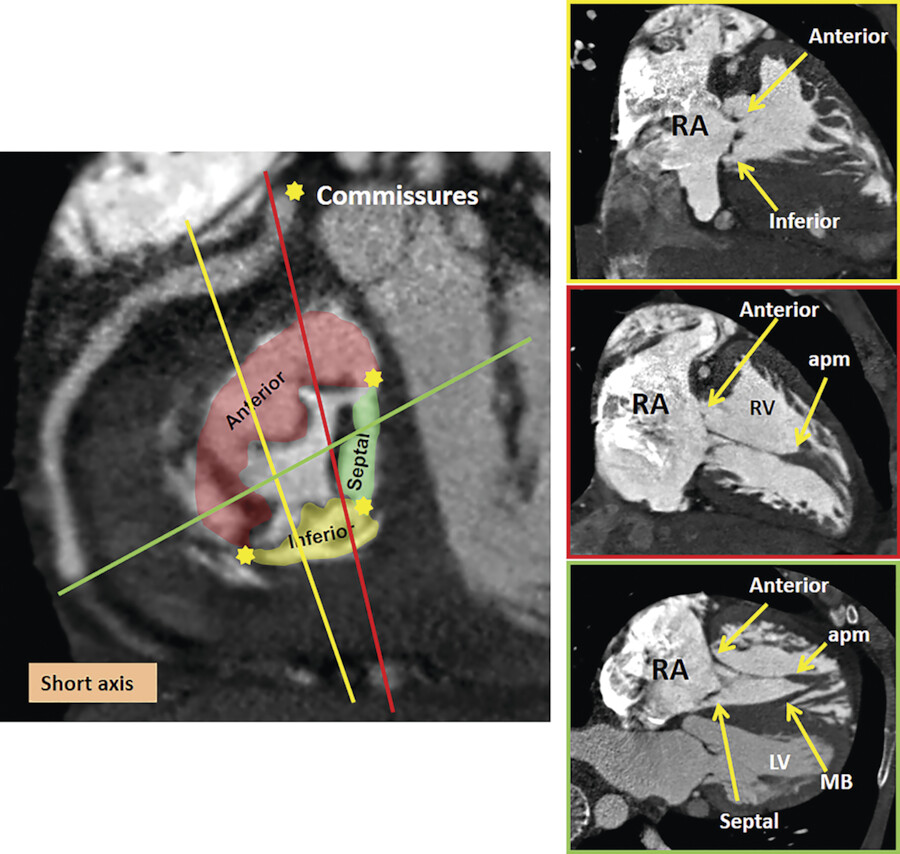
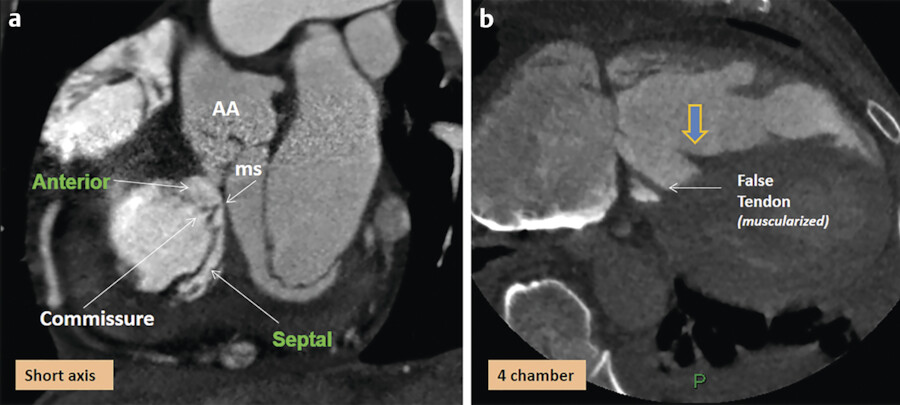
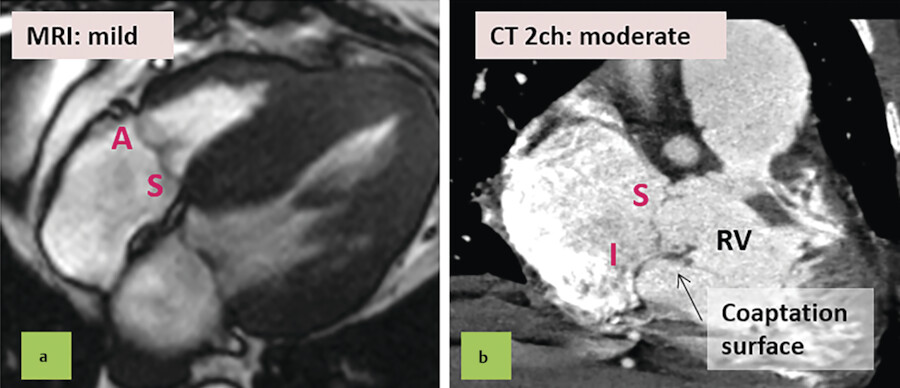
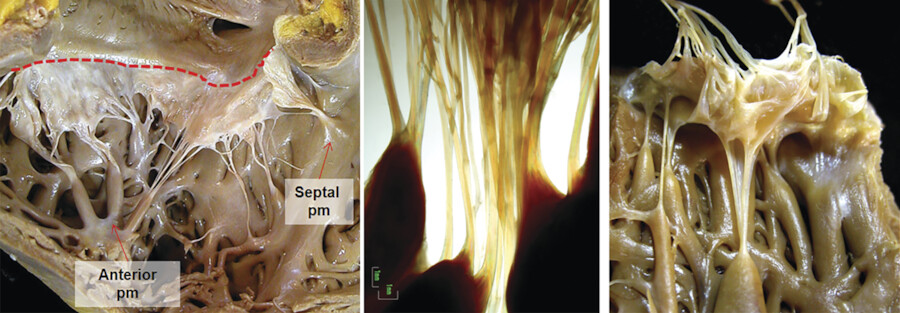
The TV apparatus consists of the three valve leaflets, the fibrous annulus, the supporting tension apparatus, which consists of the chordae tendineae (tendinous chords), and papillary muscles (PMs; Fig. 17‑2). The leaflets include anterior (superior), septal, and posterior (inferior). 6 The PMs include anterior, posterior, and sometimes a medial (septal) PM (Fig. 17‑2 , Fig. 17‑3 , Fig. 17‑4). Some believe that the TV is bicuspid in original design. 7 Embryologically, the TV has a septal leaflet and a free-wall (mural) leaflet. With each cardiac contraction, the mural leaflet tends to deform and change its shape. Therefore, to strengthen its durability, the mural leaflet has adopted to develop extra hinge point by forming a cleft within the mural leaflet that has divided it into two components, namely, the anterior and posterior (inferior) leaflets. 7 Depending on the location of the cleft (the anteroinferior commissure), the inferior leaflet can be very small. The inferior leaflet appears to be of less functional significance since it may be removed without impairment of the valve function. The septal leaflet is smaller than the anterior leaflet and arises medially directly from the tricuspid annulus above the interventricular (IV) septum. The commissure between the septal and anterior leaflets (anteroseptal commissure) is located over the membranous septum and divides it into the AV and IV components 8 (Fig. 17‑5 , Fig. 17‑6). The two leaflets may be fused at the anteroseptal commissure, but, in normal individuals, it is not uncommon to find a gap between the two leaflets at this level, in which case the gap is covered by the membranous septum. 8 In contrast to the mitral valve, the anatomical subdivision of the TV leaflets by imaging techniques appears to be less relevant since primary surgical reconstruction techniques for the TV are limited. 9 Each leaflet has an atrial and a ventricular surface. On the surface of each cord, two zones can be distinguished: a clear zone that is devoid of cordal attachments and a rough irregular zone extending between the free margin of the leaflet and the line of closure of the valve, where the leaflets coapt (Fig. 17‑7). At the coaptation area of the valve, the width of the rough zone is 7 to 8 mm and tapers toward the commissures. Tendinous cords attach to the ventricular side of the rough zone.
The anterior PM provides chordae to the anterior and posterior leaflets, and the posterior PM provides chordae to the posterior and/or septal leaflets, but variation exists. The septal PM of the conus gives chordae to the anterior and septal leaflets. In addition, there may be accessory cordal attachments to the septum, right ventricular free wall, and the moderator band (Fig. 17‑4 , Fig. 17‑6 , Fig. 17‑8). The septal PM presents in 82% of the heart and can be large in some cases (Fig. 17‑6 b). In the rest, it is replaced by tendinous chords. 10 It is a single papilla in 50% and double in 30%. The septal PM is located on the body of the septomarginal trabeculation inferior to the junction of the anterior and septal leaflets of the TV. It represents an important surgical landmark for the location of the right bundle branch (passing below the septal PM) to avoid its injury during surgical correction of certain types of VSDs.
The chordae tendineae (tendinous cords) are the thick, strong, tendinous connections between the AV valve leaflets and the PMs and help maintain the valve function (Fig. 17‑8). There are more chordae tendineae in the TV than in the mitral valve. 11 In order to perform their function efficiently, the chordae have to possess a high degree of elasticity, as well as considerable strength and endurance. The core of each cord is formed by collagen bundles covered by the endocardium composed of a superficial layer of squamous endothelial cells and an underlying dense layer of elastic fibers. Since both the valve leaflets and the chordae are composed of collagen, there is no distinguishable change in the tissue at the valvular attachment. There is variation in the origin, insertion, attachments, branching patterns, distribution, and the gross texture of the chordae (Fig. 17‑7). Commissural (marginal) cords arise as a single stem, but branch like the struts of a fan and allow the adjacent leaflets to coapt and to move apart. False chordae are seen in half of the cases, extend between PMs or a PM and the ventricle wall, and help in ventricular contraction. Rarely, cords retain their embryonic muscular structures (chordae muscularis).
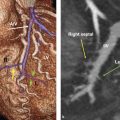
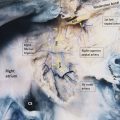
Tricuspid Annulus
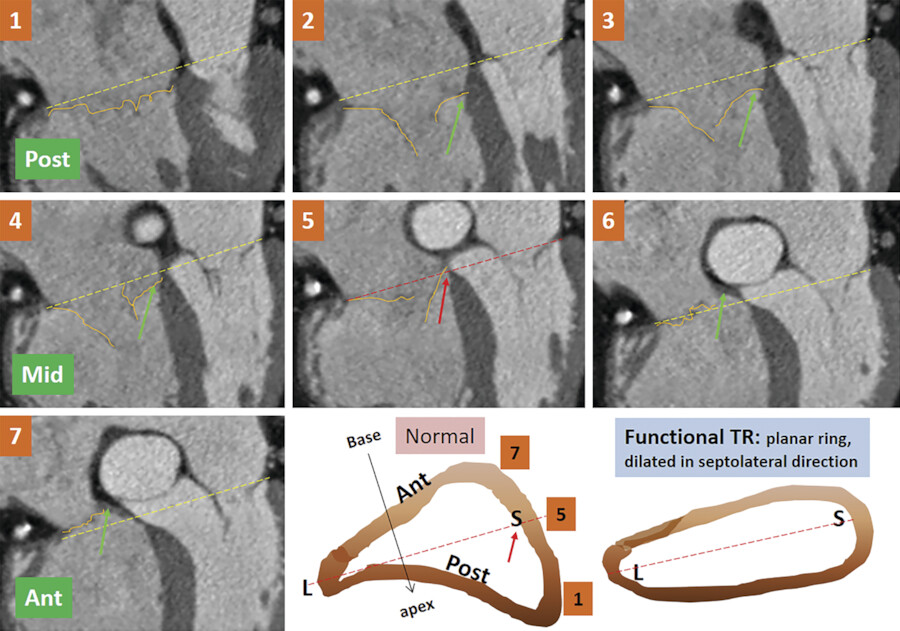
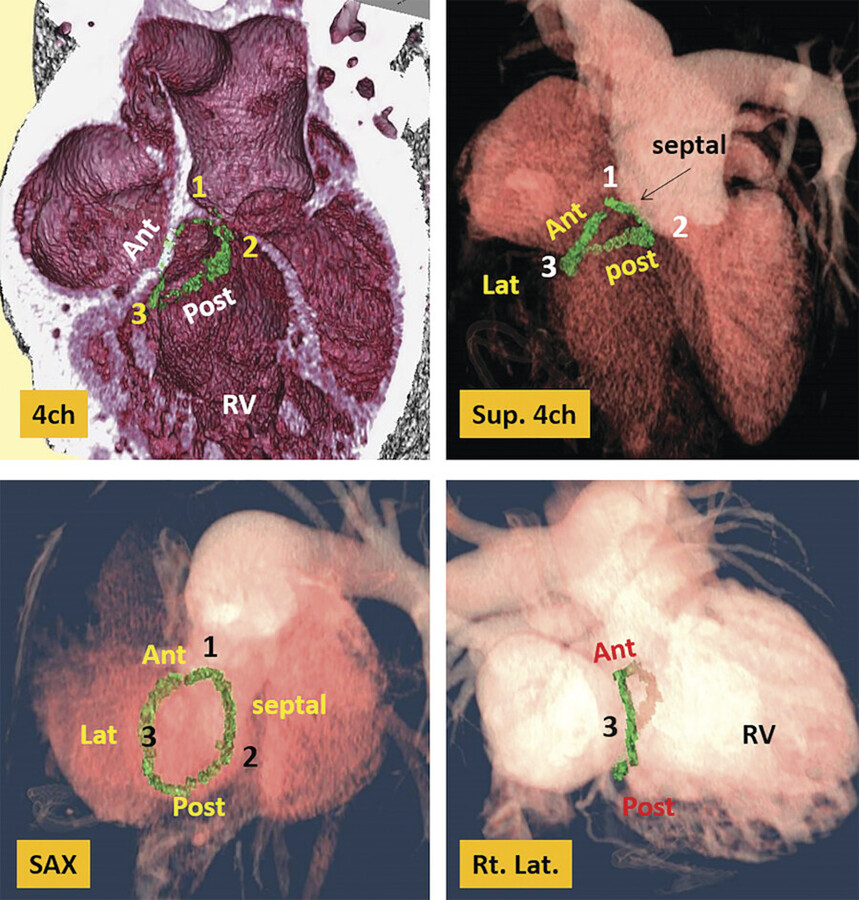
The normal tricuspid annulus has an elliptical shape and appears nonplanar, mimicking the saddle shape of the mitral valve annulus (Fig. 17‑9 , Fig. 17‑10). Common embryological origin of the two valves may explain this configuration. In an echocardiographic study by Fukuda et al 1 in healthy individuals, the tricuspid annulus was mapped throughout the cardiac cycle and reconstructed on a computer workstation. They showed a nonplanar, elliptical-shaped tricuspid annulus, with the posteroseptal portion being the “lowest” (toward the apex) and the anteroseptal portion being the “highest” (Fig. 17‑9). They suggested that future designs and techniques of TV annuloplasty should take into account not only the amount of annular dilation but also the nonplanar geometry of the TV annulus. Using, electrocardiographic (ECG) gated computed tomography angiography (CTA) images of the heart, this unique annulus morphology can be shown, provided that enough contrast exists in the right heart (Fig. 17‑9 , Fig. 17‑10). It is also possible to demonstrate the annulus changes in different phases of cardiac cycle if a retrospective data acquisition is obtained. An important consideration about the structure of the TV annulus that makes it different from the mitral annulus is the lack of extensive fibrous elements in the peripheral (mural) part of the valve in order to support its leaflets. Instead, the mural leaflets are hinged on the ventricular myocardium, with the fibrofatty tissue of the deep AV groove serving to prevent direct passage of electrical currents between the atrium and the ventricle. 8
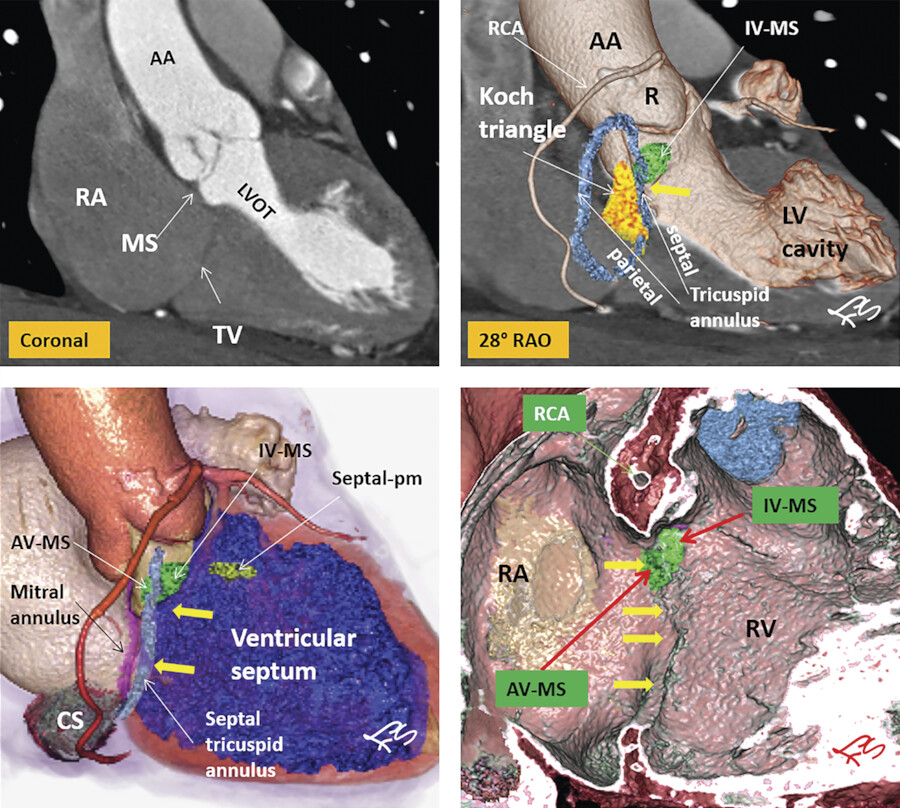
As mentioned earlier, the posteroseptal portion of the septal leaflet is the most apically displaced part. As a result of this inferior migration of the septal leaflet, the distance between the mitral valve and the TV is maximum where posterior cardiac crux is located 8 (Fig. 17‑9). In this region, also known as the muscular AV septum, the cavity of the right atrium is separated from that of the left ventricle by invagination of the AV junction myocardial walls, which is filled with epicardial fibrofatty tissue. Therefore, as emphasized by Restivo et al, this part does not appear to be a true muscular AV septum. 8 As a matter of fact, this fibrofatty space is where the AV nodal artery passes to supply the AV node. The septal leaflet of the TV also demarcates the anterior boundary of the triangle of Koch and the apex of the triangle is the membranous part of the AV septum where the bundle of His passes inferiorly. More laterally along the inferior AV groove, the septal and posterior leaflets separate the cavotricuspid isthmus part of the right atrial vestibule from the right ventricle (RV). This isthmus has an important role in the creation and maintenance of atrial flutter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree