5 Pulmonary Embolism
Summary
Pulmonary embolism (PE) remains a management challenge for the interventional radiologist. There is a spectrum of severity in patient presentation, and the long-term sequelae of PE are poorly understood. Management decisions in patients with submissive PE remain complicated due to incomplete understanding of which patients are most likely to benefit from intervention. This chapter will review the available data regarding evaluation and management of patients with PE, and will discuss the details of catheter-directed therapy in these patients. Outcomes data will also be presented.
5.1 Introduction
Pulmonary embolism (PE) is a major cause of death among hospitalized patients and is considered to be the third most common cause of death in this population. Massive PE carries a mortality rate that can exceed 58% and is typically fatal within 1 hour of presentation. 1 As a result of this major public health concern, the U.S. Surgeon General has issued a call to action to improve outcomes for patients with PE. 2
Management of PE includes anticoagulation alone, inferior vena cava filtration, adjunctive systemic thrombolysis, catheter-directed thrombolysis (CDT), catheter-directed thrombectomy/thromboaspiration, and/or surgical thrombectomy. The Antithrombotic Therapy for VTE guidelines suggest systemic thrombolytic therapy using a peripheral vein over CDT (Grade 2C level of evidence). 3 The authors comment that patients who have a higher risk of bleeding with systemic thrombolytic therapy, and who have access to the expertise and resources required to do CDT, are likely to choose CDT over systemic thrombolytic therapy. For patients with acute massive PE who have a high bleeding risk, have already failed systemic thrombolysis, or are in shock that is likely to cause death before systemic thrombolysis can take effect (e.g., within hours), catheter-assisted thrombus removal is suggested over no intervention (Grade 2C). 3 In response to the range of therapies, clinical presentations, and comorbidities that face physicians who treat PE, local multidisciplinary pulmonary embolism response teams (PERTs) have been created in order to implement these guideline suggestions and recommendations and to fill in management gaps left by uncertainties in these guidelines. A common PERT mission statement is to manage the intricacies of PE by engaging multiple specialists to deliver coordinated care to PE patients. While the PERT model is gaining a foothold in the medical community, its efficacy has yet to be determined.
5.2 Case Vignette
5.2.1 Patient Presentation
A 23-year-old female with no past medical history is brought in by emergency medical services for possible seizure/syncope. The patient states that she was standing on the escalator and started feeling short of breath, lightheaded, and hot. She syncopized and woke up on the ground. The patient also endorsed dyspnea on exertion for a couple of days during short trips to the bathroom.
She has no prior history of cancer. There is no personal or known family history of clotting disorders. The patient has been taking oral contraceptive pills for 5 years. She has no history of smoking. The patient denies fevers, chills, headache, chest pain, palpitations, abdominal pain, diarrhea, constipation, or dysuria.
5.2.2 Physical Exam
Temperature: 36.7°C; heart rate: 87 bpm; blood pressure: 111/53 mm Hg; respiratory rate: 20 per minute; pulse oximetry: 99% on RA.
General: No apparent distress; alert and oriented; well appearing.
Eyes: External ocular motion intact; pupils equal, round, reactive to light and accommodation.
CV: Regular rate and rhythm; no murmurs.
Lungs: Clear to auscultation bilaterally.
Gastrointestinal: soft; nontender.
Head/neck: nontender neck; atraumatic.
Pelvis/back: stable.
Extremities: no deformity; no calf tenderness.
Skin: no edema.
Neuro: alert; cranial nerves II–XII intact; motor WNL; sensation intact.
5.2.3 Noninvasive Testing
Troponin I: 0.2 ng/mL (normal range 0–0.4 ng/mL).
B-type natriuretic peptide (BNP): 175 pg/mL (normal range ≤100 pg/mL).
D-dimer: 2.91 μg/mL (normal range 0.27–0.5 μg/mL FEU).
5.2.4 Imaging
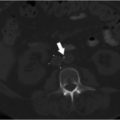
CT chest (Fig. 5.1):
Extensive pulmonary emboli extending from the distal right and left main pulmonary arteries into multiple lobar, segmental and subsegmental branches predominantly in the bilateral lower lobes.
Findings suggestive of right heart strain with right ventricle:left ventricle (RV:LV) ratio of 1.3.
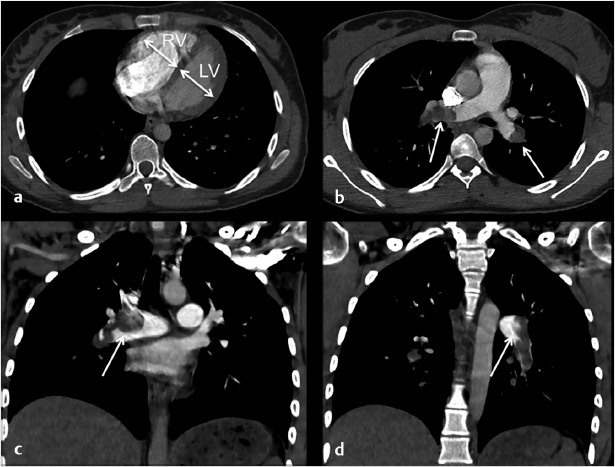
Fig. 5.1 Axial (a, b) and coronal (c, d) CT images of the chest following contrast injection. (a) A dilated right ventricle with an RV:LV ratio of 1.3. (b–d) Large filling defects in the main pulmonary arteries (arrows).
Transthoracic echocardiogram:
Dilated RV.
Reduced RV function.
Severe tricuspid regurgitation.
Severe pulmonary hypertension.
Systemic venous hypertension.
Normal LV diastolic relaxation.
Dilated right atrium.
Flattening of the septum consistent with RV overload.
CT head:
No CT evidence of acute intracranial abnormality.
5.2.5 Specifics of Consent
Prior to starting the procedure, the nature, purpose, risks, and alternatives of the procedure were explained to the patient, and informed consent was obtained. Procedure-related risks discussed included, but were not limited to, vessel injury during catheterization and major bleeding including life-threatening hemorrhage requiring transfusion and intracranial bleeding.
5.2.6 Details of Procedure
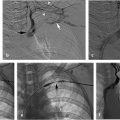
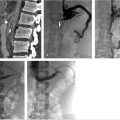
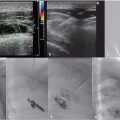
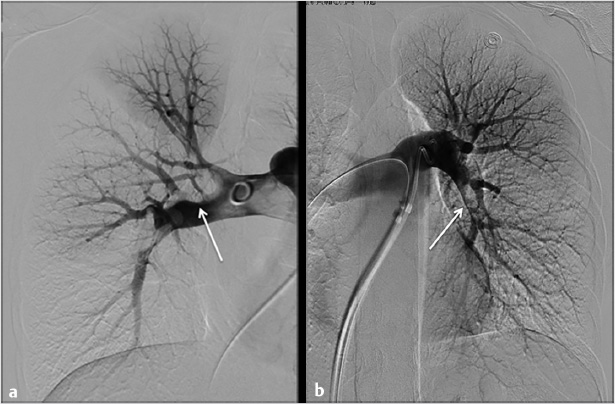
Using ultrasound guidance, the right common femoral vein was accessed at two sites and long vascular sheaths were inserted. A 7-French modified Grollman catheter was advanced into each pulmonary artery. Pressure measurements were 59/19 mm Hg (mean: 33 mm Hg) in the right pulmonary artery and 68/22 mm Hg (mean: 40 mm Hg) in the left pulmonary artery. Digital subtraction pulmonary arteriography was performed (Fig. 5.2). Based on the findings, UniFuse catheters (Angiodynamics, Latham, NY) were inserted into the largest clot-bearing branches (Fig. 5.3). Alteplase infusion was initiated without a bolus at 0.5 mg per hour through each catheter and continued for 23 hours at which time the infusion was discontinued and repeat pulmonary arteriography was performed prior to catheter removal (Fig. 5.4). Follow-up pulmonary pressure measurements were 33/13 mm Hg on the right and 29/8 mm Hg on the left.
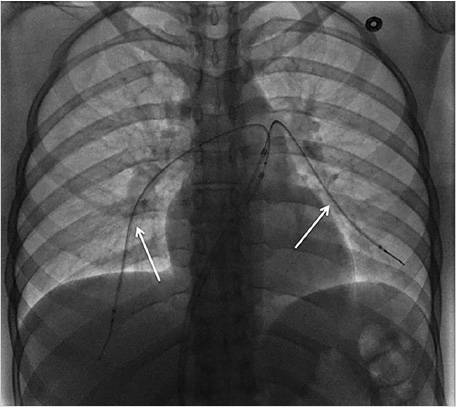
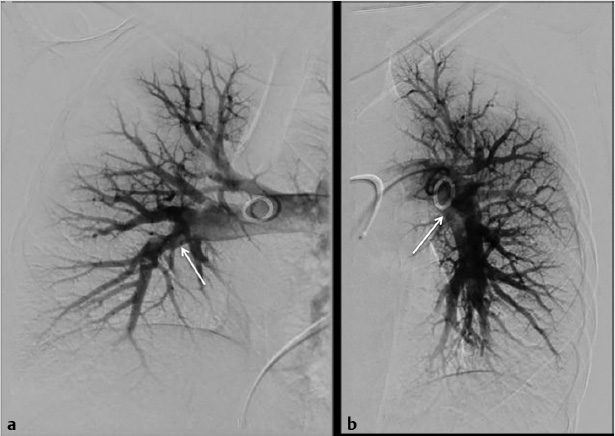
5.2.7 Follow-up
The patient initially returned for follow-up 1 month following CDT and stated that she no longer had any chest pain or shortness of breath. The chest pain stopped about 3 weeks after her procedure. She was able to attend the gym and run on the treadmill for 1 mile at a slow pace without symptoms. By her 2-month follow-up visit, she was able to run 6 miles without symptoms. She was treated with apixaban for anticoagulation without bleeding complications. An echocardiogram performed at 3 weeks postprocedure showed a dilated RV with normal function. A repeat echocardiogram at 5 months postprocedure showed normal RV size and function.
5.3 Epidemiology and Scope of the Problem
Venous thromboembolism (VTE), encompassing both deep vein thrombosis (DVT) and PE, is a common diagnosis that carries a risk of both short- and long-term morbidity and mortality. The annual event rate of VTE from 1985 to 2009 was shown to be 142 per 100,000 persons, with 30% occurring as a result of PE and 20% with concurrent PE and DVT. 4 In 2008, the U.S. Surgeon General estimated that over 100,000 deaths from PE occur yearly in the United States, and named PE as the most preventable cause of death in hospitalized patients. 2 The reason for this call to action is that in hospital mortality from acute PE still approaches 7% overall and 32% in patients with hemodynamic instability. 4 The long-term consequences of PE manifest as cardiopulmonary dysfunction and/or decreased exercise tolerance, and 1.4 to 4% of survivors develop chronic thromboembolic pulmonary hypertension (CTEPH). 4 , 5 Late recurrence of VTE has been up to 13% at 1 year, 23% at 5 years, and 30% at 10 years if not treated with extended anticoagulation. 5 Survivors face a higher overall mortality rate up to 30 years later, and PE is a significant cause of death. 6
5.3.1 Patient Presentation and Evaluation
Patients with PE most often present with dyspnea, chest pain, leg swelling, and/or dizziness. 1 , 7 The onset of symptoms can be acute or insidious (over a period of days to weeks). Other nonspecific signs/symptoms of PE include tachypnea, tachycardia, palpitations, lightheadedness, fever, cough, wheezing, and rales. In the study evaluating the patients treated by the Massachusetts General Hospital (MGH) PERT, 74% of PE patients presented with dyspnea and 33% presented with chest pain. Tachycardia occurred in 57% of PE patients, while tachypnea and hypoxemia occurred in 37 and 55%, respectively. Syncope, a sign suggestive of hemodynamic distress, occurred in 12% of this patient cohort. 7
The history, imaging, and laboratory biomarker assessment are critical upon arrival to stratify patients by their PE-related mortality. According to the American Heart Association (AHA), the major categories of patients diagnosed with PE are low-risk, submassive, and massive. 8 Patients with a low-risk PE are normotensive, have normal biomarker levels, and have normal RV function. These patients carry a short-term mortality risk of less than 1%. 8 Submassive PE refers to acute PE without systemic hypotension, but with either RV dilation, dysfunction, or myocardial necrosis. RV dilation can be determined by CT or echocardiography and is defined as an RV:LV ratio greater than 0.9. 9 RV dysfunction is defined by RV wall movement on echocardiography, BNP elevation (>90 pg/mL), N-terminal pro-BNP elevation (>500 pg/mL), or electrocardiogram (ECG) changes of new complete or incomplete right bundle branch block, anteroseptal ST elevation/depression, or anteroseptal T-wave inversion. Myocardial necrosis is defined by elevation of troponin I (>0.4 ng/mL) or elevation of troponin T (>0.1 ng/mL). Approximately one-quarter of hemodynamically stable patients will fall into this group and have a mortality risk of 3 to 15%. 10 Massive PE is the most critical category and carries a mortality rate that ranges from 25 to 65% in recent studies. 8 The criteria for massive PE include acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requirement of inotropic blood pressure support), pulselessness, or persistent profound bradycardia (heart rate < 40 bpm with signs and symptoms of shock).
In addition to the AHA guidelines above for classification of PE, clinical scoring systems have been developed to attempt to risk stratify patients, including the Wells score, Geneva score, Pulmonary Embolism Severity Index (PESI) score, and simplified PESI (sPESI) score. The PESI score is validated for the prognostic assessment of patients suffering from PE and identifies patients at low risk for 30-day mortality. 5 The sPESI has also been validated for predicting 30-day mortality and a score of 0 is accurate for identifying low-risk PE patients. 11 The European Society of Cardiology guidelines differentiate PE by mortality risk into high risk, intermediate-high risk, intermediate-low risk, and low risk. 5 Patients with PESI class III–V have a 30-day mortality up to 24.5% 12 and those with a sPESI ≥ 1 have up to 11%. 13 Patients who have a PESI class III–V or sPESI ≥ 1 without both imaging and laboratory signs of RV dysfunction can be classified as intermediate-low risk and will likely not require escalation of therapy. 5 The intermediate-high risk group merits close monitoring for rescue reperfusion therapy if hemodynamic compromise develops.
5.3.2 Preparation for Procedure
Several options exist for catheter-based treatment of PE including CDT with or without ultrasound assistance, catheter-directed mechanical fragmentation, percutaneous thromboaspiration, and percutaneous endovascular thrombectomy (e.g., AngioVac, Angiodynamics, Latham, NY). The choice of procedure and tools will differ based on the severity of the PE; mechanical fragmentation, thromboaspiration, and thrombectomy are usually reserved for patients in extremis or for those who are not candidates for fibrinolytic medications.
CDT involves the insertion of multiside hole infusion catheters into the thrombus within the pulmonary arteries. The site for access is most commonly either the common femoral vein or the right internal jugular vein. Two access sites and vascular sheaths are usually necessary if bilateral pulmonary artery infusion catheters are planned. If the common femoral vein is selected as the access site, long sheaths (55–70 cm) are required to traverse the pulmonic valve and allow for stability of the infusion catheters for the duration of thrombolysis. Options for infusion catheters include the Cragg-McNamara Valved Infusion Catheter (Covidien, Plymouth, MN), Uni*Fuse Infusion Catheter (Angiodynamics, Latham, NY), and Ultrasound-assisted EkoSonic Endovascular System (EKOS, Bothell, WA). The recommended concentration for t-PA is 0.01 to 0.05 mg/mL and can be infused at rates starting at 0.5 to 2 mg/h. 14 The EkoSonic Endovascular System (EKOS) is an FDA-cleared drug delivery catheter that uses ultrasound delivered through the catheter core. The ultrasound energy is hypothesized to alter the local architecture of the fibrin clot to improve fibrinolytic efficiency and efficacy.
In cases of massive PE, catheter-directed mechanical fragmentation or thromboaspiration may improve pulmonary perfusion and increase left heart filling pressures. When performing fragmentation, the most commonly employed technique is the rotating pigtail. 1 However, clot fragmentation can lead to distal embolization of clot fragments that can cause acute changes in pulmonary pressures and hemodynamics. Preparation for thromboaspiration in conjunction with this technique may be beneficial. The Angio-Jet Rheolytic Thrombectomy (ART) System (Boston Scientific, Marlborough, MA) has been associated with complications of bradycardia, arrhythmia, heart block, hemoglobinuria, renal insufficiency, major hemoptysis, and procedure-related death. 1 As a result, the FDA has issued a black box warning for use of this system in the pulmonary arterial system. New clot aspiration/thrombectomy tools such as the Indigo Cat8 (Penumbra, Inc, Alameda, CA), Flowtriever (Inari Medical, Irvine, CA), and AngioVac (Angiodynamics, Latham, NY) are on the horizon, but have not been formally assessed for safety and efficacy.
Therapeutic anticoagulation does not need to be discontinued prior to the procedure. Supine positioning during these procedures can be challenging due to dyspnea induced by lying flat. In these circumstances, it is important to work together with the nurse, anesthesiologist, if present, and technologist to determine the best position for the patient, which may affect the access site chosen. It is critical to understand that sedation and anesthetic agents can reduce preload and lead to cardiovascular collapse. In cases where anesthesia is necessary, involvement of a specialized cardiac anesthesiologist is recommended.
An assessment of bleeding risks and contraindications to thrombolysis is mandatory. The possibility of blood transfusion should be discussed with all patients prior to initiating thrombolysis. The components of the assessment should include the following questions. If the answer is yes to any of the questions, performance of thrombolysis should be reconsidered or not performed.
History of recent major surgery, cataract surgery, trauma, cardiopulmonary resuscitation, obstetrical delivery, or other invasive procedure within 10 days. Yes or no?
History of recent active bleeding within 3 months. Yes or no?
History of stroke, intracranial/intraspinal bleeding, tumor or vascular malformation. Yes or no?
History of cancer. Yes or no? If yes, appropriate workup for the possibility of intracranial metastases should be performed.
History of allergy to heparin, t-PA, or contrast. Yes or no?
History of an arterial puncture in a noncompressible site within 21 days. Yes or no?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree