Clinical Applications of IVIM MRI to the Nervous System
3.1 Introduction
Intravoxel incoherent motion (IVIM) refers to translational movements within a given voxel, which result in a distribution in position during measurement time [1] and can be measured using appropriate encoding gradients. The main application of this method was found to be the measurement of the restriction of thermal diffusion through the biological environment, using the concept of apparent diffusion coefficient. In particular, the observation that acute brain ischemia lesions [2] show a restriction in diffusion compared to normal parenchyma has been a tremendous breakthrough in the diagnosis and management of this disease.
Interesting information on cell density can also be extracted from the apparent diffusion coefficient (ADC), with important implications in cancer imaging, permitting the recognition of lesions with increased cellular density, such as lymphoma [3]. The ADC also permits the evaluation of the maturation of myelination processes and disease thereof in the white matter of newborns [4]. Diffusion is a three-dimensional process and varies in space according to direction because of the presence of anisotropic obstacles, which limit molecular movement, such as the axon of neurons. This led to the introduction of diffusion tensor imaging [5] and to the possibility of following noninvasively fiber tracks in the brain in vivo, assuming that the direction of fastest diffusion is followed.
There is an additional very interesting property of this method, which is the observation that incoherent motions are also produced by blood components flowing in the microvascular network [6]. This implies that perfusion information can be obtained from a few diffusion-weighted images, without contrast injection, using a biexponential signal equation model to separate perfusion effects from thermal effects, as follows:
where D stands for the diffusion coefficient, D* for the pseudodiffusion coefficient, b for the b value, and f for the perfusion fraction. This so- called IVIM signal equation model can be seen as a two-compartment extension of the ADC single-exponential signal decay model. In the brain, where this method was first applied, this model is valid for b values between 0 and 1000 s/mm2. At b values above 1000 s/mm2, additional effects due to motion restrictions of water molecules through biological barriers such as cell membranes and organelles are visible, resulting in non-Gaussian diffusive behavior [7].
In practice, multiple b value images are acquired, usually around 10 to 30, and the IVIM biexponential signal equation is fitted, typically by using a two-step method. First, a single-exponential equation is fitted for b values above the threshold of 200 s/mm2, which is the threshold above which the perfusion effects on the IVIM signal is thought to be vanishing. In a second step, the full biexponential signal equation is fitted. Interestingly, several reports [8–10] suggest that using only a few b values, even as few as two nonzero b value images, might be sufficient to extract clinically significant perfusion information in many organs, suggesting that only a minimal amount of additional scan time to a standard diffusion-weighted acquisition is necessary to obtain additional perfusion information. In the brain, because the IVIM perfusion signal is only a small percentage of the total “diffusion signal” in a voxel, around 5%, data should be acquired at the optimal signal-to-noise ratio and with a maximal reduction of susceptibility and motion artifacts.
The perfusion fraction f relates to the microvascular blood volume, or, to be more exact, to the “incoherently flowing” blood volume. D* holds information on blood speed and should be understood as the measured diffusion coefficient of the microvascular compartment, which also includes thermal motion of the blood constituents. Finally fD*, which is the multiplication of the perfusion fraction and the pseudodiffusion coefficient, is thought to be related to blood flow [11] or, in other words, to the quantity of blood flowing through a unit tissue per unit time. The perfusion fraction maps are generally less noisy in the brain compared to the D* and fD* maps, and for this reason, most clinical studies have concentrated on this parameter.
The sources of incoherent motion in a biological tissue can be multiple, and the IVIM perfusion parameters should be interpreted with this knowledge in mind. For example, in the brain, partial volume between the cerebrospinal fluid compartment and brain cortex might lead to an intravoxel biexponential behavior because the apparent diffusion coefficient of the cerebrospinal fluid is larger than that of the brain. Cerebrospinal fluid pulsation itself can lead to incoherent motion, most prominently in the sulci in the sylvian cistern and at the level of the foramen of Monro (Fig 3.1). The cerebrospinal fluid signal can be nulled using an inversion recovery pulse, such as in the widely used fluid attenuation inversion recovery (FLAIR) sequence, but due to the relative comparable T1 of blood to the cerebrospinal fluid, the signal of blood is also reduced significantly, to one-third of the total blood signal. By using a T2-prep inversion pulse compared to a conventional 180° inversion pulse, 50% more signal from blood can be recovered [12]. Such a T2-prep inversion pulse allows a significant increase in the contrast of high- grade tumors compared to the underlying normal brain parenchyma (Fig 3.2). The pulsatility properties of the cerebrospinal fluid can also be directly investigated with IVIM [13].
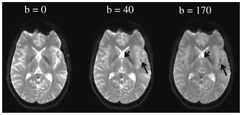
Figure 3.1 Cerebrospinal fluid pulsations can have a strong intravoxel incoherent effect. A b value of as small as 40 s/mm2 is enough to dephase most of the protons at the level of the foramen of Monro (small arrow) as well as in the sulci of the sylvian cistern (long arrow).
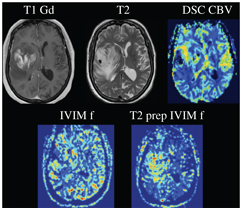
Figure 3.2 Nulling cerebrospinal fluid signal permits a significant increase in the contrast of high-grade tumors compared to the underlying normal brain parenchyma, as shown in the example of a glioblastoma centered in the right insular region. Using a T2-preparation inversion pulse permits the recovery of 50% more signal from the blood compared to using a conventional 180° inversion pulse.
The IVIM perfusion method is of interest, firstly because its methodology is essentially different than other perfusion methods, in the sense that it provides perfusion information that is specific to the local microvasculature, and the method might therefore be additional and complementary to other perfusion methods. Several recent reports have indeed been published suggesting that IVIM might provide additional perfusion information not available with other techniques about diseases such as vasospasm after subarachnoid hemorrhage (SAH) [14], cerebral small vessel disease [15], or anterior ischemic optic neuropathy [16] (see below for more details). Further, it permits the acquisition of perfusion maps without the need to inject a contrast agent, which is of particular interest given the recent discoveries of gadolinium deposition in various part of the brain after repeated intravenous (IV) gadolinium- based contrast injection [17].
3.2 Validations Studies of IVIM Perfusion Imaging in the Brain
Several important validation studies of the IVIM perfusion method have been performed and all of those studies showed positive results. First, in two independent experiments by Neil and Ackerman [18] and by Henkelman et al. [19], blood of rats was replaced with a 19F-containing perfluorocarbon blood substitute, which does remain purely intravascular in the brain because it does not cross the blood– brain barrier, and a pseudodiffusion signal was measured in the brain with fluorine nuclear magnetic resonance (NMR). In addition, under hypercapnia, which can be experimentally induced by inhalation of a gas mix with an increased amount of carbon dioxide and with the well-known effect of dilatation of the brain vessels, an increase in IVIM perfusion parameters could be observed in both rats [20] and humans [21]. Interestingly, the IVIM perfusion parameters have also been found to be dependent on the cardiac cycle and to correlate with blood speed measured with phase contrast in a proximal brain artery [22]. Finally, an effect on the signal at low b values was visible in the primary visual cortex after a visual activation paradigm [23, 24], similar to functional imaging with blood-oxygen-level-dependent magnetic resonance imaging (BOLD MRI) and in accordance with the well-known local increase in blood flow under specific tasks (for more details on this topic, see Chapter 5, on fMRI IVIM).
A simple, linear relationship between the standard perfusion parameters and the IVIM perfusion parameters can be derived, assuming a random capillary network and a sufficiently long diffusion time [11] (for more details, see Chapter 1, “Introduction to IVIM MRI”). Using this relationship, several studies found a fair to good correlation between the perfusion fraction f and blood volume as measured with the clinically most used MRI perfusion method dynamic susceptibility contrast [25]. The results concerning the correlation of the other perfusion parameters, that is, between IVIM and dynamic contrast–enhanced perfusion parameters as well as arterial spin labeling are currently less conclusive, in part because of the sparse number of studies on a range of organs and pathologies and in part due to contradictory results of the published studies [25]. Certainly, additional work on the exact relationship between the IVIM perfusion parameters, the microvascular network structure, and the blood flow and blood speed is necessary. Indeed, many additional factors might be relevant, such as the viscosity of blood, turbulent and pulsating flow, flow restrictions through vessel wall components, and variability of the capillary network structure. Finally, a study comparing directly the IVIM perfusion method to an accepted quantitative gold standard perfusion method, such as positron emission tomography (PET) or single-photon emission computed tomography (SPECT), would be of great interest.
3.3 Clinical Application of IVIM Perfusion Imaging in the Brain
The potential clinical applications of IVIM in the brain include all perfusion-related diseases. In addition, because of its particular properties compared to other perfusion methods, IVIM might provide specific microvascular perfusion information not available otherwise, which could be of particular interest in diseases concerning the microvasculature.
3.3.1 Stroke
Perfusion imaging plays a major role in therapeutic decision making in the context of acute stroke. It is currently thought to be the best method to evaluate the tissue at risk to die if nothing is done to salvage this tissue, which is also called the penumbra. The infarct core, that is, the irreversibly damaged brain parenchyma, can also be assessed with perfusion imaging. Whether IVIM perfusion imaging can play a role in therapeutic decision making in stroke remains to be established, but the property of the IVIM perfusion method to be essentially local, in the sense that because both excitation and readout are done in the same plane, is particularly interesting in cases of slow blood flow. This might be particularly useful in cerebral stroke or severe carotid artery stenosis, in which local blood flow might be nevertheless conserved through appropriate collaterals. In three independent studies, a significant decrease in f was measured in the infarct core compared to the contralateral hemisphere in acute stroke [26–28] (Fig 3.3). It is important to note that in regions where the perfusion compartment is vanishing, caution is advised when interpreting D*, because D* becomes the pseudodiffusion coefficient of an “empty compartment” and is therefore not properly defined [28]. In interesting preliminary results in hyperacute stroke imaged within 6 h after symptom onset, the IVIM microvascular perfusion was found to be retained in the penumbra (infarct core excluded) compared to the healthy contralateral side but was decreased in the infarct core, demonstrating that microvascular perfusion is retained through collateral flow in the not-yet-infarcted tissue at risk [29]. This suggests that IVIM perfusion imaging might be a marker of the quality of the collateral blood flow in the penumbra in hyperacute stroke patients.
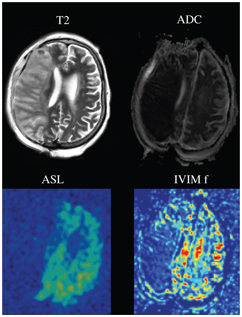
Figure 3.3 Acute stroke of the territory of the right middle cerebral artery, with a clear hyperintense signal on the T2-weighted image (T2), and a reduced apparent diffusion coefficient (ADC). The perfusion of the right middle cerebral artery territory is clearly vanishing, as demonstrated with both method arterial spin labeling cerebral blood flow (ASL) and IVIM perfusion fraction f.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree