Stage
T category
N category
M category
Dukesa
MACa
0
Tis
N0
M0
–
–
I
T1
N0
M0
A
A
T2
N0
M0
A
B1
IIA
T3
N0
M0
B
B2
IIB
T4a
N0
M0
B
B2
IIC
T4b
N0
M0
B
B3
IIIA
T1–T2
N1
M0
C
C1
T1
N2a
M0
C
C1
Any T
N1c
M0
C
C1
IIIB
T3–T4a
N1
M0
C
C2
T2–T3
N2a
M0
C
C1/C2
T1–T2
N2b
M0
C
C1
IIIC
T4a
N2a
M0
C
C2
T3–T4a
N2b
M0
C
C2
T4b
N1–N2
M0
C
C3
IVA
Any T
Any N
M1a
–
–
IVB
Any T
Any N
M1b
–
–
Table 19.2
Prognosis based on AJCC staging classification of colon and rectal cancer distributed by American Cancer Society (results from study of National Cancer Institute’s SEER database on 120,000 people diagnosed with colon cancer between 1991 and 2000)
Colon stage | 5-Year survival rate (%) | Rectal stage | 5-Year survival rate (%) |
|---|---|---|---|
I | 93 | I | 90 |
IIA | 85 | II | 70 |
IIB | 72 | III | 56 |
IIIA | 83a | IV | 7 |
IIIB | 64 | ||
IIIC | 44 | ||
IV | 8 |
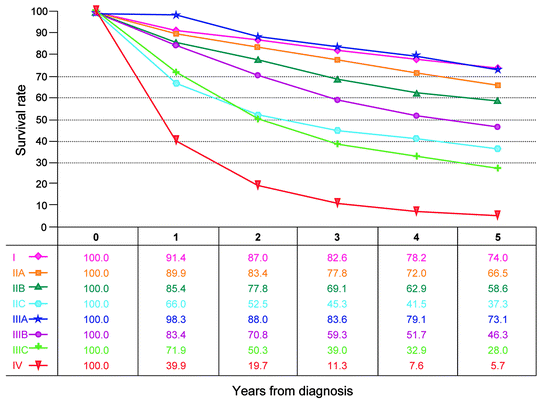
Fig. 19.1
Observed survival rates for 28,491 cases with adenocarcinoma of the colon. Data from the SEER 1973–2005 Public Use File diagnosed in years 1998–2000. Stage I includes 7,417; Stage IIA, 9,956; Stage IIB, 997; Stage IIC, 725; Stage IIIA, 868; Stage IIIB, 1,492; Stage IIIC, 2,000; and Stage IV, 5,036 (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition [2010] published by Springer Science and Business Media LLC www.springer.com)
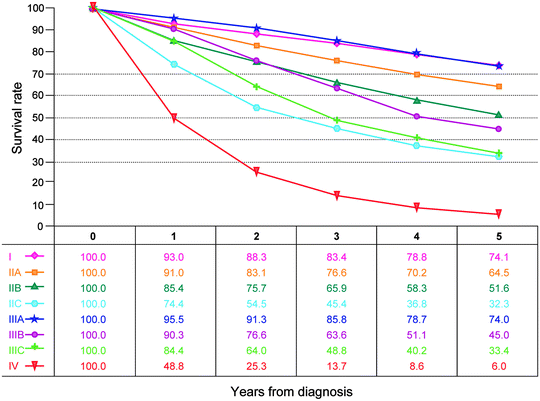
Fig. 19.2
Observed survival rates for 9,860 cases with adenocarcinoma of the rectum. Data from the SEER 1973–2005 Public Use File diagnosed in years 1998–2000. Stage I includes 3,470; Stage IIA, 2,752; Stage IIB, 165; Stage IIC, 268; Stage IIIA, 595; Stage IIIB, 615; Stage IIIC, 761; and Stage IV, 1,234 (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition [2010] published by Springer Science and Business Media LLC www.springer.com)
Clinical Objectives in Colorectal Cancer
The first objective in managing colorectal cancer patients is adequate and complete preoperative staging, which is routinely done by abdominal and thoracic CECT to evaluate overall liver status. The purpose of primary tumor treatment is to be as curative and radical as possible, while exactly defining local disease extension. This also needs to be obtained in cases of isolated metastatic spread. Surgery is usually the first choice of treatment for localized disease and single and/or resectable metastases. In local extended disease, the use of neoadjuvant chemoradiation therapy appears to improve prognosis [19, 20]. Adjuvant treatment is indicated to limit tumor recurrence, based on initial tumor extension and prognostic factors (stage III, lymph node metastases, poorly differentiated tumors, lymphovascular invasion), and it ordinarily consists of systemic chemotherapy protocols based on 5-fluorouracil as first choice. On completion of treatment, close follow-up is essential because of the high percentage of disease recurrence after primary treatment (30–40%) [21]. Follow-up entails systematic evaluation by morphological imaging techniques (CT, abdominal ultrasounds) and systematic evaluation of serum markers (CEA) to detect relapse or metastatic spread. Recurrence can be local, regional (lymph node localizations), peritoneal seeding or metastatic liver/lung lesions, and it is closely correlated with primary tumor characteristics. The recurrence rate in locally extended tumors is about 20% and rises to around 50% in the presence of initial lymph node involvement.
Current Role of Nuclear Medicine
Of the nuclear imaging modalities for managing patients with colorectal cancer, [18F]FDG-PET is becoming the most widely used. It is considered the most useful technique for achieving clinical objectives and has been added to standard imaging techniques as a new “strategic” tool in this scenario. Currently, [18F]fluorodeoxyglucose ([18F]FDG) is the most useful and commonly used metabolic tracer in oncology.
Several studies have demonstrated that whole body [18F]FDG-PET is an accurate noninvasive technique in staging/restaging several types of malignancies, and its usefulness has also been proved in the management of patients with colorectal cancer [22–24]. Guidelines on the appropriate use of [18F]FDG-PET in cancer recognize an appropriate use of this technique in restaging patients with suspected recurrence of colorectal cancer, elevated serum tumor markers such as CEA, and a negative or inconclusive standard diagnostic work-up, and in presurgical evaluation of patients with recurrence of disease and potentially resectable metastatic lesions [25]. In the preoperative initial staging of disease, [18F]FDG-PET is considered potentially useful but not yet sufficiently demonstrated [25].
Finally, [18F]FDG-PET in colorectal cancer holds promise for systematic follow-up and evaluation of response to therapy, especially in the evaluation of chemoradiation therapy efficacy in metastatic cancer (late and early response) or of local treatment efficacy such as radiofrequency ablation (RFA) of liver metastases. Furthermore, because positivity and intensity of [18F]FDG uptake are an expression of tumor aggressiveness, [18F]FDG-PET is also considered as a prognostic tool.
Classic, standard nuclear imaging techniques such as 99mTc-HDP/MDP bone scan in disease staging and restaging are limited to the evaluation of secondary bone localizations. Finally, nuclear techniques such as 90Y-microsphere treatment for unresectable liver metastases are becoming increasingly available. The current and potential uses of nuclear medicine techniques are discussed in the following sections.
Presurgical Staging of Primary Colorectal Cancer
Because few studies on a small number of patients are currently available, the role of [18F]FDG-PET in the presurgical staging of primary disease remains controversial. Primary cancer is detected and studied by morphological imaging, oral contrast CT, and endo-ultrasonography, which also allows for biopsy and histological confirmation. Several studies reported a high sensitivity of [18F]FDG-PET (95–100%) in detecting the primary tumor also when in situ [26–28]. The tumor’s histopathological features and lesion diameter are closely correlated with these data. False-negative results have been reported in cases of mucinous carcinoma and of small tumor foci in tubulovillous polyps or villous adenoma [26, 27, 29]. Abdel-Nabi et al. reported false-positive PET findings [positive predictive value (PPV) 90%] in patients without colorectal cancer but with IBD or previous diagnostic polipectomy [26]. Although [18F]FDG-PET appears to be more useful in detecting regional lymph node involvement and liver metastases, conflicting study results have been reported. Abdel-Nabi and Kantorova both found higher sensitivity (78–88%) with PET than with contrast-enhanced abdominal/pelvic CT (38–67%) or ultrasonography (25%), with high specificity (96–100%) in detecting liver metastases [26 27]. Furokawa reported that, when compared with multidetector helical CT for routine staging, [18F]FDG-PET did not seem superior in terms of sensitivity and accuracy [28]. In this study, [18F]FDG-PET accuracy in detecting lymph node involvement did not show a statistical difference in comparison with CECT accuracy (59% vs. 62%). Other studies confirmed low PET sensitivity (around 30%) primarily due to false-negative findings in cases of micrometastases or the presence of metastatic lymph nodes adjacent to the primary tumor [30]. In a review by Vriens et al. other more recent studies in small groups of patients showed that [18F]FDG-PET can change patient management in 12–27% of cases when added to CT and/or pelvic magnetic resonance imaging (MRI) and ultrasonography, leading in the bulk of cases to cancelation of surgery after unexpected metastatic lesions were detected, or to extension of the surgical plan or the radiotherapy field, or to institution of neoadjuvant treatment after the detection of pathological lymphadenopathy missed at morphological imaging [31–34]. In brief, the weighted mean change in the management of colorectal cancer calculated in the review was about 10.7% (95% confidence interval [CI] 7.6–14.5%) [35]. The discordant findings among the different studies can be explained by the patient selection bias, which showed a major impact of [18F]FDG-PET in patients with a high metastatic potential, while in localized disease [18F]FDG-PET gave less additional information to the standard diagnostic work-up (contrast-enhanced CT and colonoscopy). What can be said at present is that the use of PET in staging primary colorectal cancer can lead to a change in clinical management when compared to standard diagnostic work-up, but its systematic use in this application is not yet recognized.
Recurrent Colorectal Cancer
The suspicion of colorectal cancer recurrence is oftentimes prompted by a rise in serum marker values (CEA) or abnormal findings at anatomical imaging (CECT, MRI) during follow-up and/or occurrence of new symptoms. [18F]FDG-PET remains the mainstay of nuclear imaging in the follow-up of patients with colorectal cancer. In cases of elevated serum CEA values and negative morphological imaging findings, [18F]FDG-PET is advised because of its ability to detect early disease and to reveal metabolic changes in normal-sized structures before morphological findings appear. Data published so far show that in about two of three cases whole body [18F]FDG-PET identifies recurrence of disease, making its use in an early phase of patient follow-up recommended [36]. Flamen et al. showed that [18F]FDG-PET can detect disease recurrence in more than 80% of patients (43/50) [37]. In this study, disease recurrence missed at morphological imaging was located in the liver (27%), locally (20%), the lung (9%), other abdominal sites (36%), and other extra-abdominal non-pulmonary lesions (9%). These results are consistent with both previous and more recent studies [38–40].
[18F]FDG-PET is recommended when indeterminate lesions at conventional morphological imaging need to be characterized in order to differentiate disease recurrence from scar tissue [41–44]. Identification of presacral recurrences in particular, which develop in a high percentage of patients, poses a considerable clinical challenge. Assessment with conventional pelvic imaging studies [CECT, transrectal ultrasound (TREUS)] is problematic for differentiating postsurgical or radiotherapy residual fibrotic tissue from disease recurrence; in the latter case, further treatments should be considered (Fig. 19.3). Flamen et al. showed that [18F]FDG-PET offers additional diagnostic value in 56% of cases compared to contrast-enhanced CT alone and in 20% of cases compared to contrast-enhanced CT in combination with TREUS [45]. Even-Sapir et al. demonstrated that PET/CT in patients with colorectal cancer who underwent abdominoperitoneal or anterior resection had 98% sensitivity, 96% specificity, a 90% PPV, a 97% NPV, and 93% accuracy in distinguishing benign from malignant presacral abnormalities [46]. In recurrence confirmed at morphological imaging, [18F]FDG-PET is recommended to complete disease staging, because it can identify additional unexpected metastatic sites (upstaging) compared to CECT alone (Fig. 19.4) [47]. The general usefulness and the additional diagnostic value of [18F]FDG-PET for this purpose was demonstrated in a 2000 meta-analysis by Huebner et al. that showed that [18F]FDG-PET leads to a change in clinical management in about 30% of patients with recurrent colorectal disease when added to standard imaging techniques in the evaluation of this patient subset [47]. In another study, Flamen et al. evaluated [18F]FDG-PET and CECT performance in 103 patients with suspected recurrence of colorectal cancer [45]. [18F]FDG-PET showed higher sensitivity than CECT in detecting metastatic lymph nodes in the abdominal cavity negative at CECT, especially those located in retroperitoneal and mesenteric sites. A statistically significant additional value of [18F]FDG-PET was also found in the evaluation of extra-abdominal regions, where it identified unexpected metastases, most of which were located in the lung. The literature reports discordant results for the evaluation of liver involvement. In the study by Flamen et al. no additional value of [18F]FDG-PET in terms of sensitivity was found compared with normal CECT and/or MRI findings, but PET did allow to correctly classify anatomically undefined liver lesions [45]. Truant et al. showed that the sensitivity of [18F]FDG-PET was equivalent to that of contrast-enhanced CT for hepatic sites (79% for both), and highly superior for extrahepatic abdominal sites (63% vs. 25%) [48]. A 2002 meta-analysis demonstrated that [18F]FDG-PET can also be superior to conventional diagnostic techniques (CT, ultrasonography, and MRI) in the detection of liver metastases (sensitivity around 90%) and that it can be considered as the most sensitive noninvasive imaging modality for the detection of hepatic metastases arising from gastrointestinal tract (GI) tumors, especially in colorectal cancer [49]. An interesting study by Sobhani et al. on 130 randomized patients undergoing complete follow-up (physical examination, biomarker assay, conventional imaging, and [18F]FDG-PET) found that the time to recurrence detection was shorter for the patients studied by [18F]FDG-PET than those who underwent conventional imaging (12.1 vs. 15.4 months), which led to the possibility of initiating a more curative treatment [50]. In a large group of patients (n = 115) presenting with recurrent colorectal cancer, Valk et al. reported that PET had a global sensitivity of 93% and a global specificity of 98% in detecting metastatic sites, compared with 69% and 96%, respectively, for CECT alone, confirming that [18F]FDG-PET should be routinely performed in the follow-up of these patients. The most relevant finding to emerge from this study was that PET identified unexpected metastases in 29% of patients presenting with only one site of recurrent disease at CECT, leading to an upstaging of disease [38]. Several studies later evaluated the impact of [18F]FDG-PET or [18F]FDG-PET/CT on the management of patients presenting with potentially curable liver metastases who underwent PET for complete restaging of disease [51–54]. In this patient subset, PET allowed complete preoperative staging, determination of whether other neoplastic foci were present, and the choice of the most adequate treatment, thus avoiding unnecessary surgery. Lai et al. demonstrated that PET identified unexpected metastases in 25% of patients referred for presurgical staging to evaluate liver metastases resectability [51]. A 2005 review by Wiering et al. including all studies that had evaluated patients referred for presurgical staging for resectable liver metastases showed [18F]FDG-PET had sensitivity and specificity rates for liver lesions of 88% and 96.1%, respectively, and 91.5% and 95.4%, respectively, for extrahepatic lesions [55]. [18F]FDG-PET resulted superior to CT in all cases but overall in detecting extrahepatic lesions. The change in patient management was about 32%, with cancelation of surgery and planning of systemic chemoradiation therapy in most cases. The review by Vriens et al. of 25 papers found a pooled mean management change of 22.3% in 1,060 patients [35]. With the introduction of combined PET/CT, the evaluation of many tumors and of colorectal cancer as well, has improved since it allows correlations between abnormal tissue metabolic changes detected at PET and anatomical structures defined at CT, with accurate localization and characterization of lesions [56–58]. Cohade et al. showed how in-line PET/CT improves diagnostic accuracy of PET alone in restaging recurrent colorectal cancer, especially in the evaluation of abdominopelvic extrahepatic disease, thus reducing false-positive findings due to unspecific or inflammatory [18F]FDG uptake [59]. In this study, the use of PET/CT instead of PET alone improved diagnostic accuracy from 78 to 89%. A review evaluating the potential of PET/CT in comparison with CECT, MRI, and PET alone suggested that, when available, [18F]FDG-PET/CT appears to be the diagnostic tool of choice in early evaluation of recurrent colorectal disease [60]. Furthermore, the use of low-dose CT scanning to correct PET emission images for attenuation (instead of radioactive sources, as in the past) shortens the time needed to complete whole body acquisition [61].
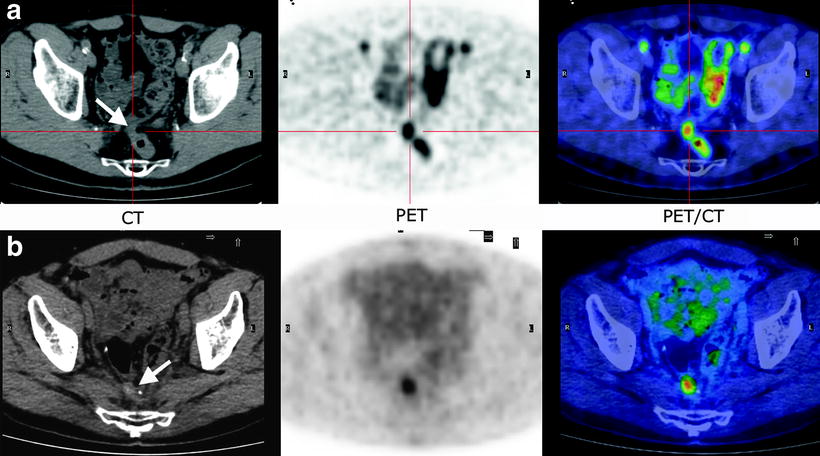
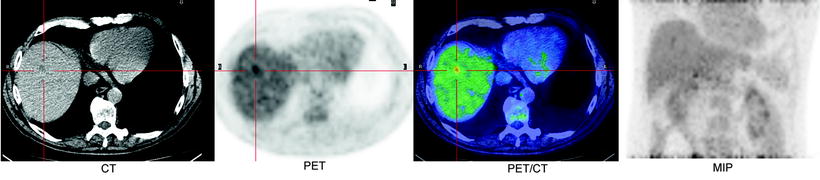

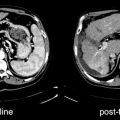
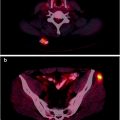
Fig. 19.3
Metabolic characterization of undetermined presacral lesions by [18F]FDG-PET/CT. The nature of the lesions was confirmed by histological evaluation after the PET study. CT (a and b): suspected, undetermined presacral lesions at CT (arrows); PET scan shows (a and b) intense [18F]FDG uptakes at the lesions level indicating disease recurrence; FUSION: Overlap of CT and [18F]FDG-PET images allows to easily localize and to characterize [18F]FDG uptakes

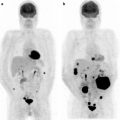
Fig. 19.4
Patient treated for primary colorectal cancer and referred for restaging. PET scan identified unexpected metastases in the liver not seen at CECT
Still unclear is the influence of [18F]FDG-PET on disease-free survival and overall survival in patients with recurrent colorectal cancer, owing to the difficulty with comparing studies of different patient subgroups with different treatment plans; nonetheless, whole body [18F]FDG-PET has a key role in clinical practice and provides additional value to standard diagnostic work-up and a high clinical impact. In this subset of patients, [18F]FDG-PET should be considered as an essential tool for better clinical management. Given its high NPV (around 95%), when a PET scan in this patient subgroup is negative, the presence of detectable disease recurrence could be excluded with [18F]FDG-PET, though close clinical follow-up should still be undertaken. While there is considerable scientific evidence for the usefulness of [18F]FDG-PET, certain limitations to the technique deserve mention.
[18F]FDG-PET can produce false-positive findings at evaluation of abdominal recurrence when postsurgical inflammation and inflammatory disease are present (i.e., abscesses, colitis, and rectal fistula). The physiological [18F]FDG uptake in the GI and genitourinary tracts due to the excretion of the tracer itself can mimic and also hide pathological sites.
The risk of false-negative findings is high in the presence of miliary liver metastatic spread, due to the physiological uptake of [18F]FDG in the liver parenchyma and to a low lesion to background ratio, or low [18F]FDG-uptake in diffuse peritoneal effusion.
Besides anatomical sites, lesion diameter is another important factor affecting PET accuracy and may be responsible for false-negative results, especially in lymph node or hepatic lesions <1 cm in diameter, this being near the technique’s lower limit of spatial resolution. Finally, high patient blood glucose levels (>150 mg/dL) can deteriorate imaging quality, and some metastatic lesions, especially those in the liver, can be missed [62]. This is why blood glucose levels should be accurately evaluated, and at least 6 h fasting before scanning is required. A meta-analysis by Huebner et al. evaluated the influence of false-positive and false-negative results on [18F]FDG-PET sensitivity and specificity in patients with recurrent disease [47]. The final data showed that false positives had a greater impact than false negatives. In fact, the sensitivity of whole body [18F]FDG-PET resulted high (97%), with similar rates for the detection of liver (91–96%) and pelvis (94%) involvement. Specificity values in the evaluation of recurrence differed for total body (76%), liver, and pelvis (97–99%) due to the greater likelihood of false-positive results in extrahepatic and extrapelvic regions than in isolated organs. Furthermore, a study by Akhrust et al. [63] on a group of patients who underwent [18F]FDG-PET for presurgical staging demonstrated that sensitivity was lower in those who received neoadjuvant chemotherapy pretreatment due to the risk of the stunning phenomenon that leads to false-negative results when [18F]FDG-PET is performed too early after the end of treatment.
Treatment Response Evaluation
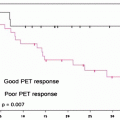
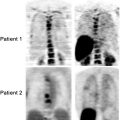
The identification of responders to chemotherapy is of interest for selecting patients who may be expected to benefit from continued treatment and for selecting those who could be treated with other drugs. Evaluation of response to treatment is ordinarily by morphological assessment of target lesions and of changes in lesion diameter overtime. Currently, the Response Evaluation Criteria in Solid Tumors (RECIST) are the most widely used set of rules to define disease response to treatment: complete response is defined as disappearance of target lesions at morphological imaging; partial response, a minimum reduction of 30% in lesion diameter; disease progression, a minimum increase of 20% in lesion diameter or appearance of new lesions; and stable disease, neither partial response nor disease progression [64]. With [18F]FDG-PET came the need to have similar criteria for metabolic response, but consensus is still lacking. A significant decrease or increase in [18F]FDG uptake in target lesions during treatment has always been taken as sign of treatment response or disease progression, respectively; but lacking standardized limits and standardized timing of response assessment, each study uses its own criteria. One limitation to [18F]FDG-PET is its inability to detect minimal residual disease below the range of system spatial resolution. [18F]FDG uptake is detectable in lesions measuring 5–10 mm in diameter, which correspond to about 108–109 tumoral cells. But even with this limitation, a negative PET scan during or at the end of treatment is predictive of good prognosis since it indicates disease response. Furthermore, the time to negative results on PET scans during treatment is a prognostic factor and a predictive element for final tumor response. If after 2 cycles of chemotherapy a PET scan is negative, as demonstrated in lymphomatous disease, the chances of obtaining remission at the end of the treatment are high, whereas the chances of remission with a few more chemotherapy cycles are lower if PET scans taken early at the beginning of treatment persist positive [65–67]. Treatment response evaluated by [18F]FDG-PET is clearly related to a better overall survival and disease-free survival in most types of tumors. Metabolic response to treatment is normally evaluated quantitatively by measuring variation in SUVmax (standardized uptake value), which is a more practical and reproducible way than with qualitative visual methods, even if many studies have employed the latter, with good stratification of subsequent prognosis [68–71]. Quantitative evaluation has to be reproducible, which means that pre-therapy and post-therapy scans have to be made in the same way, with the same scanner, similar injected activity, and the same patient preparation (weight and glycemia) [72]. The problem is to determine which percentage in SUVmax drop is to be considered significant for defining responder or nonresponder with prognostic meaning. Many studies have proposed their own cut-off values (25–35%) for the drop in SUVmax [73, 74]. Recently, Wahl et al. have proposed the Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) as standardized criteria to define metabolic response to treatment. These criteria include standardized patient preparation: fasting at least 4–6 h before injection, serum glucose <200 mg/dL, insulin administration before [18F]FDG-PET not indicated, image acquisition obtained 50–70 min after injection and reconstructed with the same software for baseline and posttherapeutic scan, with up to 15 min between the acquisition of the two scans, and SUVmax corrected for lean body mass as calculated by an ROI (region of interest) system of detection. Furthermore, it is recommended that up to five target lesions are chosen, two for organs with the highest [18F]FDG uptake, better if about 2 cm in diameter. These target lesions will usually correspond to the target lesions considered by RECIST. Response to therapy is evaluated as a continuous variable expressed as the drop in the percentage of SUVmax between the pre- and posttherapeutic scans. Complete metabolic response is defined as the disappearance of all metabolic active tumor, with the target lesion showing the same uptake as the liver and indistinguishable from the surrounding background; a partial response, as a decrease of >30% in SUVmax of the most intense lesion; progressive disease, an increase of >30% in SUVmax of the most intense lesion or a visible increase in the extent of [18F]FDG uptake.
Still debated is the number of chemotherapy cycles after which [18F]FDG-PET has to be performed to evaluate the response and the delay in time between the last treatment and [18F]FDG-PET; the PERCIST committee considers them as treatment-specific criteria. Several studies demonstrated that SUVmax begins to drop after only one cycle of chemotherapy, at which time early response can be detected [74, 75]. Based on previous studies, especially in lymphomatous disease, the suggested delay between the end of the treatment and the final [18F]FDG-PET is around 10 days to 3 weeks for chemotherapy and 3 months after radiotherapy to avoid the stunning risk and the occurrence of false-positive results [76]. [18F]FDG-PET has been demonstrated to be a useful tool to evaluate tumor response to treatment and to stratify patients for prognosis also in colorectal cancer [77]. Several studies have demonstrated that there is an interest in performing [18F]FDG-PET to evaluate response to neoadjuvant treatment, i.e., chemoradiation therapy with cytoreduction intent before surgery, during chemotherapy in advanced metastatic tumor, and after local treatment of liver metastases to detect complete/incomplete treatment. Evaluation of response to radiotherapy is very difficult by anatomic imaging alone because residual tissue persists after irradiation, making it impossible to reliably differentiate by CT or MRI (accuracy 30–60%) between residual neoplastic disease and fibrosis [78–80]. [18F]FDG-PET, however, can detect residual active disease and assess metabolic response in the persistence of anatomic abnormalities, address surgical planning and optimize the technique, thus reducing surgical invasiveness if tumor response has occurred, or leading to a more aggressive approach in the absence of response. A review by Wahl et al. of 19 studies published between 1992 and 2008 reported an NPV for [18F]FDG-PET of 83–100% and a PPV of 77–100%, depending on the different criteria used to define the response [64]. All these studies differed in the definition of response criteria, the delay between treatment and [18F]FDG-PET acquisition, and clinical endpoints (metabolic response and histological verification vs. overall survival and disease-free survival). The studies taking histological confirmation of [18F]FDG-PET findings as an endpoint demonstrated a significant correlation between [18F]FDG-PET residual uptake and histological response (viable tumor cells). In the majority, a decrease in SUVmax after a median of 3–6 weeks after irradiation seemed to predict good response to radiotherapy. Most of the studies proposed quantitative evaluation of tumor response by using a decrease in SUVmax or SUVmean after treatment, but with different cut-off values (30–60%); others proposed kinetic models that, although reproducible, are more difficult to perform in clinical practice. The study by Melton et al. in particular, which compared quantitative methods [decrease in SUVmax >70% and decrease in total lesion glycolysis (TLG)] versus qualitative visual methods, showed that for the evaluation of response to neoadjuvant treatment for colorectal cancer the quantitative is more accurate than the qualitative method [81]. Of note, however, is the risk of false-positive results due to post-irradiation inflammatory processes and false-negative results due to minimal residual viable disease under the detection limits of [18F]FDG-PET. A decrease in the SUVmax in patients undergoing treatment is not only the expression of tumor response, and the SUVmax itself is not only the expression of tumor absolute [18F]FDG avidity: both need to be interpreted for their prognostic meaning. Some studies demonstrated that patients considered responders to [18F]FDG-PET after chemoradiation therapy of the primary tumor had a better median overall survival and disease-free survival [71, 82–85]. Other studies demonstrated that the absolute SUVmax or SUVmean can stratify patients with a better or worse overall survival, but at which cut-off is not yet clear. Calvo et al. for example, showed that patients with a pre-therapeutic tumor SUVmax ≤ 6 had a better overall survival at 3 years after neoadjuvant and surgical treatment than those with a higher tumor SUVmax (92% vs. 60%) [86]. Riedl et al. proposed different ranges of SUVmax with different prognosis as expressed by median overall survival [87]. The role of [18F]FDG-PET in the evaluation of systemic chemotherapy in advanced metastatic tumors is also under evaluation. A recent review by De Gesus Oei gives an overview of the results of five studies [88–93]. All the studies underlined the importance of detecting early response to treatment after a few cycles of chemotherapy to differentiate responder patients from nonresponders in order to optimize treatment. The problem is to define the correct timing for [18F]FDG-PET so as to obtain a good correlation between global response and prognosis. These studies compared [18F]FDG-PET findings at 1 or 2 weeks after the start of chemotherapy and then at 1–3 months. The majority demonstrated that the clinical correlation between metabolic response and treatment outcome was better detected at 1–3 months after the start of chemotherapy. Here, too, quantitative evaluation by SUVmax or SUVmean is considered a better way to evaluate metabolic response than qualitative assessment. Furthermore, Dimitrakopoulou et al. demonstrated that the absolute SUVmean of the most avid lesions at pre-therapeutic PET could predict response to treatment, a second-line chemotherapy in this study, sustaining the concept that the higher the [18F]FDG avidity as expressed by SUVmax or SUVmean, the more resistant the lesions are to treatment [91].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree