Chapter Outline
Computed tomography colonography (CTC) is a low-dose, cross-sectional imaging examination optimized for the detection of colorectal polyps and masses. As a result of advanced computer 3D postprocessing, a popular misperception is one of an imaging analogue to optical colonoscopy in which a 3D model is used to view the mucosal surface of the interior of the colon. However, CTC is fundamentally a different examination, using 2D images and 3D datasets in the interpretation of mucosa, deeper structures, and even processes beyond the colon. Since its introduction in 1994, CTC has matured into a clinically effective modality. Central to this transition are technologic advances in computer hardware and software that have allowed the easy acquisition and manipulation of large volumetric CT data sets. Performance in low polyp prevalent screening populations has now been validated in a number of prospective clinical trials, with a general consensus that CTC performs substantially better than double-contrast barium enema and is equivalent to optical colonoscopy for clinically relevant polyps and masses.
This chapter will provide a comprehensive overview of this modality. The technical components that comprise the examination, including bowel preparation, colonic distention, and image acquisition, as well as alternative CTC approaches such as noncathartic or laxative-free (previously mislabeled as “prepless”) protocols, will be discussed. Interpretation will be covered in detail, including common strategies currently in use. The mechanics of interpretation will be highlighted, with coverage of the more common and important pitfalls that can affect the accuracy of this process. The indications and uses for CTC will be examined, outlining the optimal screening target and the polyp-carcinoma sequence that forms the underlying rationale of the selective polypectomy strategies for CTC-based screening. In addition to screening, diagnostic applications for CTC and the evaluation of colorectal cancer (CRC) staging will be covered. Finally, pertinent issues central to CTC will be examined, including radiation dose, complications, and extracolonic (incidental) findings.
Technical Components
CT colonography is a multicomponent examination that is undertaken over several days. The major technical components include bowel preparation, colonic distention, and image acquisition. A programmatic or team approach is favored because it allows each portion of the procedure to be optimized for reproducible, high-quality examinations. Thus, a nurse program coordinator can answer patient questions on bowel preparation in the days prior to the scan to minimize preparation failure, and the technologist can optimize the bowel distention while the patient is on the scanner table. The radiologist can interpret the examination and forward the results for further coordination of care to be undertaken by the program nurse. Such division of labor allows the radiologist to concentrate totally on interpretation. Without a team approach, the technical components are often addressed in a less complete fashion. A breakdown in any study component ultimately limits the potential impact of the overall examination.
The traditional approach to CT colonography involves a fully cleansed and tagged colon. Alternate methods have been investigated, including laxative-free techniques, but large multicenter validation studies remain to be done. Once the colon has been prepared, it is distended with gas (carbon dioxide or room air) and finally imaged with a low-dose, thin collimated technique in a minimum of two patient positions. The following sections outline the principles and major strategies in use for the technical components of the examination. In addition, common problems that may arise will be addressed. The protocol at the University of Wisconsin (UW) is highlighted as one specific solution.
Bowel Preparation
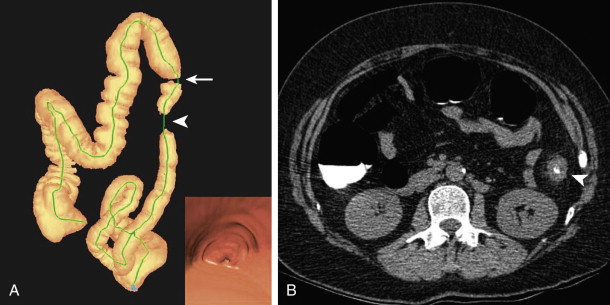
The objective of this first portion of the examination is to prepare the colorectum for optimal imaging. Traditionally, this involves oral administration of a cathartic agent to purge the colon of fecal matter that could mimic or obscure a colonic polyp. Tagging agents are also administered orally to admix with any remaining stool and colonic fluid. Contrast agents increase the attenuation of stool and fluid to allow easy differentiation of soft tissue polyps from stool and detection of polyps submerged in fluid ( Fig. 53-1 ). Bowel preparation is typically undertaken 1 to 2 days prior to the scheduled examination. The specific agents and protocol used in bowel preparation often vary among different institutions.
Cathartic agents have traditionally been classified as dry or wet preparations. Both types are in use with CTC. Dry preparations result in only minimal residual colonic fluid and include low-volume osmotic cathartics, such as magnesium citrate; wet preparations often result in larger amounts of residual fluid, occupying up to 50% of the lumen in various segments. Wet preparations include polyethylene glycol (PEG) and formulations based on PEG. Early on, there was an emphasis placed on using dry cathartic preparations with CTC to minimize obscuring polyps by residual fluid. At this time, tagging agents were not typically used, and any unopacified fluid would obscure a submerged polyp. Although the polyp should theoretically be seen when the patient was imaged in a complementary position (i.e., supine vs. prone), it could be missed when detected only on one view, and the examination became considerably more tedious and difficult to interpret because of the difficulty in determining which portion of the colon mucosa had been interrogated and what remained to be seen. With the addition of colonic tagging, such considerations regarding the amount of residual fluid have become less important because the polyp can be seen as a soft tissue structure in the high-density colonic fluid (see Fig. 53-1 ). On the other hand, some reports have suggested that dry formulations remain advantageous over wet preparations because they may promote the phenomenon of polyp contrast coating. There is increasing recognition that polyp coating is an important aid in polyp detection, particularly those of a flat morphology (see later, “Interpretation”).
The major cathartic agents currently in use include magnesium citrate and PEG. Magnesium citrate is an osmotic cathartic agent. It is one of the dry cathartics in which relatively small amounts are ingested and work by means of an osmotic effect. Because of its hypertonicity, fluid is pulled across the colonic mucosa into the colonic lumen. The fluid then serves as an effluent that lavages out the colon. Because of this mechanism of action, it mandates that the patient be well hydrated before catharsis; otherwise, the cleansing action is less than optimal. Furthermore, it is important to maintain hydration throughout the preparation period to maintain effective cathartic action and decrease the possibility of dehydration. Holte and associates have shown that osmotic cathartics can cause significant volume loss over the preparation period, during which a median weight loss of 1 kg was seen, despite fluid intake of 4 L in a series of healthy volunteers. Typically, a multidose magnesium citrate regimen is undertaken with CTC (e.g., two doses separated by a predetermined time interval) to allow optimal cleansing. Single-dose regimens have shown less effectiveness and more poor preparation outcomes. Magnesium citrate has long been used for barium enema examinations, and there is considerable experience with its use in this regard. It is well tolerated, with a favorable safety profile. It can be used in almost all patients aside from those with severe renal dysfunction and those with tenuous fluid balance control.
PEG is a wet-based preparation. In addition to standard PEG, several related formulations are in use with CTC, including Miralax and HalfLytely. PEG is a balanced solution with various electrolytes and a high molecular weight, nonabsorbable polymer that prevents secretion or absorption. Large volumes (4 L) are ingested to create the effluent. PEG is considered the safest of cathartics and can be used even in debilitated, fluid-tenuous patients because negligible fluid and electrolyte shifts occur across the colonic mucosa. When completed, this preparation leads to excellent catharsis in most patients. The major drawback has been compliance with the preparation because of decreased palatability. One study showed that up to 38% of patients failed to complete the entire preparation. As noted, because of its wet preparation nature, there is often a fair amount of residual colonic fluid.
Sodium phosphate is an osmotic cathartic agent that was used widely in the past but is no longer favored. It is mentioned for historical reasons and to highlight its potential dangers. Until 2008, it was one of the major cathartic agents in use for CTC and optical colonoscopy because of its excellent cleansing abilities and high compliance rate. Patients were more likely to complete the regimen, which required ingestion of a small-volume solution or ingestion of multiple pills. However, sodium phosphate had a narrow therapeutic range, with a higher incidence of complications. In 2004, a rare but generally irreversible form of renal failure was identified with sodium phosphate use mediated by a precipitation of calcium phosphate in the renal tubules (so-called phosphate nephropathy). After recognition of this potential complication, a black box warning was issued by the U.S. Food and Drug Administration in 2008, and this agent now requires a prescription. For CTC, sodium phosphate and formulations that contain sodium phosphate are typically no longer used.
Tagging agents include barium formulations and iodine-based solutions. For barium, there are two common densities used, a dilute 2% w/v preparation (CT barium) or a much denser 40% w/v preparation. The primary action of barium is to tag particulate stool internally. It admixes with the stool to increase the attenuation substantially from 40 to 60 HU to well over 200 HU, allowing for easy identification from soft tissue (see Fig. 53-1 ). Barium can also tag fluid but tends to do so inhomogeneously, with a gradient effect related to gravity, in which the more dependent portions of the fluid are more densely tagged. Iodine-based agents are ionic (e.g., diatrizoate) or nonionic (e.g., iohexol, iopamidol). The primary action is to tag residual colonic fluid (see Fig. 53-1 ). It can also tag stool but often does less well than barium-based agents in this regard. There is considerable variation in tagging approaches. Some CTC protocols use a single tagging agent (barium or iodine alone), and others use a two-agent approach (both barium and iodine) to take advantage of the benefits of each.
The UW protocol is one of several that have demonstrated excellent results for bowel preparation on a consistent basis, with nondiagnostic rates related to bowel preparation with CTC much less than 1%. The specific protocol is outlined in Table 53-1 . It is predicated on the principles of complete colonic catharsis and tagging of residual stool and fluid. The cathartic choice has been magnesium citrate because of its palatability in comparison to PEG. A double-dose regimen is required to cleanse the colon adequately. As opposed to some protocols, the cathartic is given prior to tagging (as opposed to the reverse situation) to purge the bulk of the stool and increase the effectiveness and efficiency of tagging; a wider window for optimal tagging exists for a given amount of tagging agent. With the reverse situation, larger amounts of tagging agents are needed to tag the bulk colonic contents.
| Time * | Agent | Amount | Comment |
|---|---|---|---|
| 8 am | Clear liquid diet | Maintain hydrated state. † | Beginning with breakfast |
| 11 am | Bisacodyl (Dulcolax) | 2 tablets orally ‡ | Stool softener |
| 3-5 pm | Magnesium citrate | 300 mL (one bottle) | Cathartic § |
| After 3 hours, administer | Magnesium citrate 2% w/v Barium sulfate | 300 mL (one bottle) 250 mL (one bottle) | Cathartic Tagging |
| After 3 hours, administer | Diatrizoate | 60 mL | Tagging |
| 9-11 pm | Preparation is complete for the examination the following morning. | ||
* Prior to 8 am , the patient is NPO after midnight.
† It is important to maintain hydration to optimize the cathartic action. Recommend hydration prior to cathartics and at least 4-6 cups of liquid with each of the osmotic cathartic
‡ Does not require the patient to be near a bathroom. Catharsis begins with administration of magnesium citrate.
§ If polyethylene glycol is substituted, the first dose should be given around noon (16 8-oz cups, one every 10 minutes, for a total of 4 L).
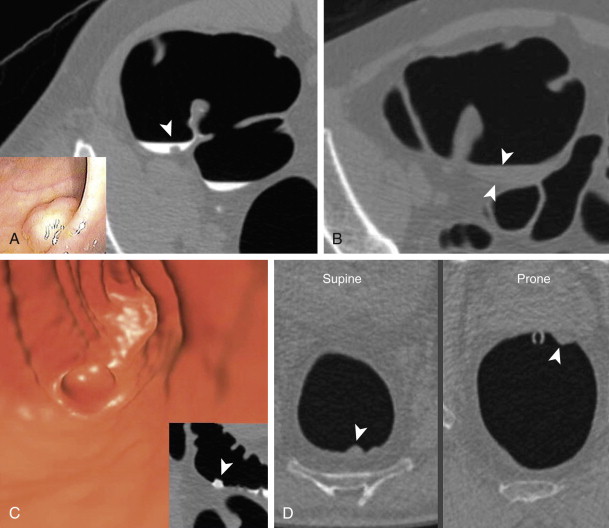
A dual tagging approach is preferred (as opposed to a single agent) to take advantage of the benefits of each agent—barium for stool and iodine for fluid. The dilute formulation of barium (2% w/v) is used in the UW protocol. The 2% version tags the residual stool particles well and is not too dense with CTC. As opposed to the denser 40% preparation, this formulation has not obscured visualization of colonic mucosa or clogged colonoscopic ports; this is important because most patients with CTC-detected polyps undergo same-day colonoscopy to take advantage of the prepped status. Regarding the second tagging agent, the ionic nature of diatrizoate is preferred over a nonionic solution, despite its less palatable taste. In addition to tagging residual fluid, the increased osmolarity of the solution works like a mild second cathartic, resulting in a final cleansing of the colon. This action is particularly important when a dry cathartic agent is used in the protocol. From the barium enema era, it was noted that use of magnesium citrate promotes excessive barium coating. In the case of CTC, without the final cleansing action of diatrizoate, this can lead a right-sided studding or even a thick film of tagged stool ( Fig. 53-2 ). This pitfall often precludes the use of 3D CTC for polyp detection in this situation (see later), which can lead to decreased sensitivity for lesion detection. It is thought that the substitution of nonionic iodine solutions could lead to a similar situation, in which the final hypertonic cleansing does not occur.
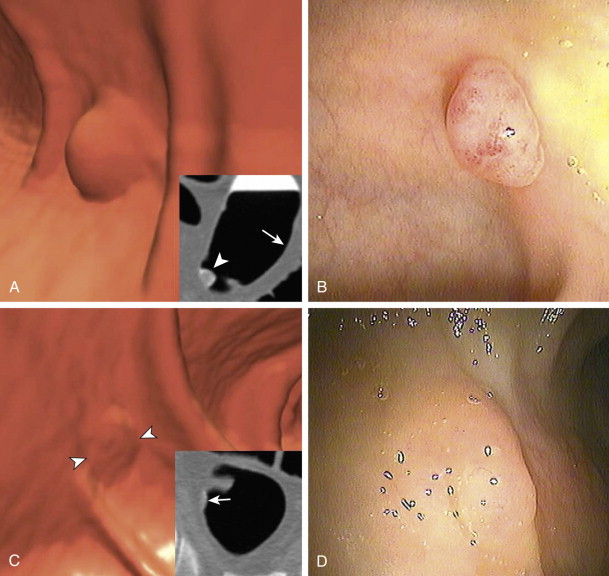
Finally, and perhaps most importantly, the combination of the three agents in this protocol (magnesium citrate, 2% barium, and diatrizoate) appears to promote a polyp-coating phenomenon ( Fig. 53-3 ). The tag appears to adhere to the mucosa overlying the polyp (CTC equivalent of a polyp etched in white at barium enema) while it is washed away from the normal colonic mucosa. It is postulated that there is an interaction between a negatively charged mucin film elaborated by polyps and the positively charged barium tag. The diatrizoate washes the tag away from normal mucosa but not from the polyp surface. This phenomenon has been very helpful in detecting polyps, particularly those of a flat nature, which are only minimally raised from the colonic surface. Whether this phenomenon is reliably seen with wet-based or iodine-only preparations is unknown.
Colonic Distention
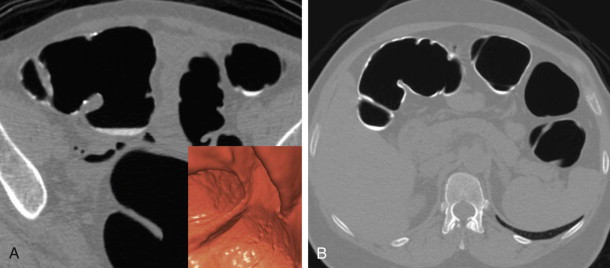
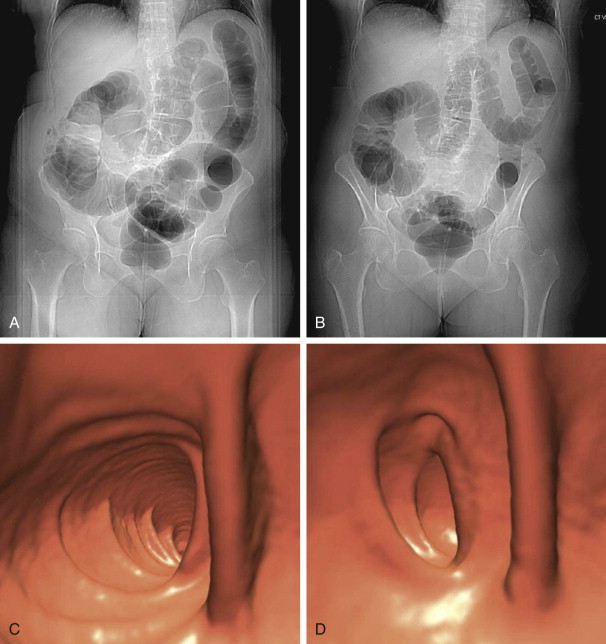
The objective of this portion of the examination is to distend the colorectum optimally to allow the easy detection of intraluminal projections and true wall thickening, but also to balance the degree of distention with acceptable patient discomfort. With optimal distention, the colonic walls are well separated; the walls are thin and almost imperceptible. Thus, polyps are well delineated as they project into the colonic lumen. The high contrast between the gas-filled lumen and soft tissue nature of the polyp allows for even many small diminutive polyps to be seen. It is important to realize that the colon does not have to be completely distended on a given series to be diagnostic in nature. It can be partially underdistended on a single series. However, the corresponding series often shows excellent distention in the same area because of the complementary shifting of gas to nondependent regions, allowing for an interpretable examination ( Fig. 53-4 ). When needed, a decubitus series can be helpful.

There are two gaseous agents available for colonic distention. Of these, carbon dioxide is favored over room air because it is actively absorbed across the colonic mucosa and eventually expelled by the respiratory system. Reduced instillation pressures essentially preclude perforation and promote patient comfort. Because the colon rapidly decompresses, the patient feels relief very quickly after the instillation ceases. In contrast, room air is composed chiefly of nitrogen, an inert gas in which there is no active reabsorption. Because of this, manual instillation pressures can be very high, with the possibility for perforation. In addition, patients can feel uncomfortable and bloated for several hours after colonic distention with room air until it is passed through the gastrointestinal (GI) tract.
The major strategies for colonic distention include manual room air insufflation and automated carbon dioxide insufflation. Room air insufflation was initially the primary method for distention with CTC. There was considerable prior experience with barium enema, and the equipment was inexpensive and readily accessible. This was undertaken with manual bulb insufflation after the insertion of an enema tip. The air was introduced by staff or by patient self-insufflation. However, there are several major problems with this CTC strategy that led to less than optimal examinations. Most importantly, unlike with barium enema, there was no real time assessment of the degree of colonic distention. With barium enema, distention adequacy could be determined at fluoroscopy. With CTC, no real-time visualization is possible; adequacy was surmised by a set number of puffs relative to patient body habitus or by patient feedback to distention discomfort. Therefore, colonic distention was variable, dependent on the administering staff and the patient’s individual perception of or tolerance to distention. Also, this was a very labor-intensive method that required individual coaching for each case. In addition to the difficulties of distending the colon, there was the possibility of perforation because fairly high wall pressures could be generated with this “blind” administration. Most institutions have since moved to automated carbon dioxide (CO 2 ) insufflation because of difficulties with room air insufflation and several recognized advantages with the use of CO 2 . However, because of the inert nature of room air, in which there is no active resorption, room air can be used to distend segments that are resistant to CO 2 .
CO 2 -based distention involves the use of an automated insufflator. Because CO 2 is actively resorbed, it requires continuous low-pressure instillation, which can only be undertaken with automated delivery. After insertion of a small-caliber, flexible-tip catheter, CO 2 is instilled at low pressures, regulated by the machine. During the filling phase, intracolonic pressures (as measured by the insufflator) generally range from 11 to 19 mm Hg. The pressures may transiently increase to 35 to 40 mm Hg with positional change or if colonic spasm occurs, but will quickly decrease as the spasm resolves. At some point, the pressures settle in a range of 20 to 28 mm Hg (with volume measurements typically at 3.5 to 4.0 L) and an equilibrium state is achieved—the CO 2 inflow balances the ongoing losses (resorption by colonic mucosa, as well as potential loss around the catheter and/or reflux into small bowel). At this point, the colon is typically optimally distended. The benefits of this equilibrium state are that it can be maintained as long as the CO 2 remains infusing, and it is easily identified by viewing pressure and volume measurements on the machine console. Thus, distention is much more reproducible and less labor-intensive with this approach. In addition, because of the low-pressure delivery, in which maximum pressures rarely exceed 50 mm Hg for a few moments, complications are almost nonexistent (see later).
The use of spasmolytics is controversial. Glucagon, a polypeptide pancreatic hormone with smooth muscle relaxant effects, has shown mixed results, without definite improvement in distention or improved patient comfort. In addition, it has been reported to increase the incidence of small bowel reflux. It is typically not used with CTC in the vast majority of U.S. institutions. However, in Europe and Canada, Buscopan (hyoscine butylbromide) is widely used. This anticholinergic agent acts on the postganglionic parasympathetic smooth muscle receptors to cause colonic relaxation. Several studies have shown increased distention and patient comfort, however, without a significant increase in polyp detection. Contraindications include a history of glaucoma. It is not available in the United States.
The UW distention protocol is outlined in Table 53-2 . Patient discomfort is minimized by setting the instillation pressure at 17 to 18 mm Hg for the average-sized individual, which is adjusted upward as needed. The key for optimal distention is to attain and maintain an equilibrium state prior to imaging. As noted, this is determined by assessing pressure and volume measurements recorded by the insufflator (21 to 29 mm Hg and 4 L, respectively). One key point to optimizing distention is related to volume. Although equilibrium pressures can be seen with volume measurements at 2 L, it has been preferable to wait until 3.5 to 4 L has been reached ( Fig. 53-5 ). In our experience, persons are often imaged too quickly before full distention because of high-volume concerns and the possibility of perforation. It is important to understand that the volume recorded by the machine does not represent the amount in the person’s colon because there is continuing ongoing loss (see earlier). Equilibrium is reached when the infusion is matched by the loss; thus, the low-pressure instillation could be extended indefinitely. As such, it is important to wait until the volume measurements reach about 4 L, even if equilibrium pressures (20 to 25 mm Hg) are attained earlier to allow full distention. Typical recorded volume measurements at the conclusion of the examination range from 5 to 6 L although it is not uncommon to have numbers in the range of 10 to 12 L. The CTC technologist is an important person in this part of the examination; real-time quality assurance checks are carried out by this trained individual to determine whether additional imaging is required (e.g., a decubitus series).
| Volume (L) | Pressure (mm Hg) | Patient Positioning and Comments |
|---|---|---|
| 0.0 | 0.0 | Insert rectal catheter with patient in LLD and inflate balloon. |
| 0.0-2.0 | 10-19 | Fill with patient in LLD. |
| 2.1-4.0 | 10-19 * | Roll RLD and continue filling. |
| 4.0+ | 20-30 | At equilibrium, turn supine to scout and scan. |
| 4.0+ † | 20-30 | Roll prone and do quality check of supine series. |
| 4.0+ † | 20-30 | Wait until equilibrium is reestablished; then scout and scan prone series. |
| 4.0+ † | 20-30 | Quality check of prone series and determine need for decubitus series |
| 6.0-8.0 ‡ | 20-30 | Typical volume and pressure at end of examination |
* May rise to 20 to 30 mm Hg suggesting equilibrium but continue to fill until volume reaches 3.5 to 4 L. If pressures rise to 30 to 40 mm Hg, transient spasm is likely.
† Continuous infusion throughout required to maintain equilibrium. Volume should continue to rise.
‡ May see volumes exceeding 10 L if patient has loss around catheter or incompetent ileocecal valve.
Some common distention difficulties include machine- and patient-related factors. Active problem solving, however, can often salvage an otherwise suboptimal examination. One common situation is frozen or unchanging pressure and volume measurements, in which it does not appear that carbon dioxide is infusing. The cause is an obstruction at some level, usually in the tubing. In addition to factors such as lying on the tubing, a common cause is a column of fluid in the tubing, which can block the CO 2 infusion because of the low-pressure nature of instillation. The patient will often report that the bowel preparation was started late. Once the tubing is cleared of fluid, the infusion will restart. Other situations, in which CO 2 infuses but does not optimally distend the colon, may be related to almost empty tanks or to the patient’s underlying colon and decreased distensibility from conditions such as diverticular myochosis or frank stricture.
Image Acquisition
Once the colon is optimally distended, the patient is scanned. At a minimum, two series are obtained, with the patient in supine and prone positions. Each series is undertaken in end- expiration to minimize the extrinsic compression of the lungs on the transverse colon. The datasets should be immediately evaluated to ensure that there is adequate distention and that no colonic segment is collapsed on both views. If this occurs, an additional series (typically with the patient in a right lateral decubitus position) can be undertaken.
CTC represents a low-dose, thinly collimated, and typically noncontrast examination. The optimal parameters represent a balance between maintaining polyp visualization while minimizing dose. Thus, although submillimeter collimation is possible, overlapping 1.0- to 1.25-mm slice thicknesses are adequate, given that the examination positivity size threshold is set at 6 mm for CTC. Overall, the required parameters for the examination are not stringent, and high-quality CTC results can be obtained with basic multidetector CT (MDCT) scanners. As opposed to examinations such as cardiac CT, in which high temporal and spatial resolution are needed (to image small-caliber cardiac vessels on a beating heart), the high-contrast nature of soft tissue polyps projecting into a gas-filled lumen and the static nature of the colon allow the use of standard 16-slice MDCT scanners. The scanning parameters of the UW protocol are given in Table 53-3 . Dose exposures are approximately 3 to 5 mSv for the examination.
| Parameter * | GE LS VCT 64 |
|---|---|
| Detector configuration | 64 × 0.625 |
| Rotation time (sec) | 0.5 |
| Pitch | 0.984 |
| Speed (mm/rot) | 39.36 |
| Slice thickness (mm) | 1.25 |
| Interval (mm) | 0.75 |
| kV | 120 |
| Smart mA/auto mA range | 30-120 |
| Noise index | 50 |
Alternative Protocols
In addition to the standard cathartic-tagging regimen, considerable interest has centered on alternative protocols that minimize or eliminate the cathartic portion of the bowel preparation. The cathartic requirement for CRC screening modalities has long been recognized as a major barrier in colorectal cancer screening compliance. Noncathartic or laxative-free CTC holds the possibility of substantially increasing screening rates by appealing to those who currently refuse screening because of purging requirements. Whether polyp sensitivity can be maintained in this situation is an area of ongoing research.
The typical approach of this modified CTC protocol is to use a low-fiber diet followed by the ingestion of tagging agents. Often, the protocols are multiday regimens, so the popular monikers of “prepless” or “minimal prep” CTC are a misnomer. There is a bowel preparation regimen but the cathartic aspect has been removed. The colon is distended in a fashion similar to that for standard CTC. Often, postprocessing in the form of electronic cleansing or digital subtraction of the tagged feces is used. Single-center feasibility studies have shown excellent results in high-polyp prevalence cohorts. Recently, a multicenter prospective trial in a low-prevalence screening cohort reported per patient sensitivities of 91% for adenomas at the 10-mm threshold, with decreased sensitivities of 59% at the 6-mm threshold. This study highlights the current tradeoff with noncathartic protocols. Although perhaps more appealing to patients, there is a real decrease in sensitivity for polyp detection by laxative-free CTC versus the standard cathartic and tagging protocol, particularly for smaller subcentimeter polyps. In addition, these studies are more difficult to read, requiring expertise in distinguishing a postprocessing digital subtraction artifact from subtle polyps at 3D or relying on primary 2D approaches alone.
Interpretation
Once the technical aspects of the CTC examination are complete, the datasets can be networked to a workstation to allow 2D and 3D review. The main objective of interpretation is to identify soft tissue polyps and exclude pseudopolyps, typically related to retained feces or thickened folds. There is general consensus that an unavoidable learning curve exists for CTC. CTC interpretation requires the traditional cross-sectional skills from CT as well as additional CTC-specific skills for accurate interpretation. Two major strategies were initially proposed, defined in terms of detection: (1) a primary 2D approach with 3D problem solving; and (2) a primary 3D approach with 2D confirmation. Most recently, a combined approach has been favored. All detection strategies ultimately use a common characterization pathway, and the process of interpretation (regardless of specific detection approach) can be broken down into two major tasks, as described here.
There are two general tasks of interpretation with CTC. The first is one of detection, in which focal intracolonic structures that potentially represent a polyp are identified (with 2D or 3D review). Once a list of possible polyps is established, the second task is characterizing each of these possible polyps—the soft tissue polyps are confirmed and the pseudopolyps are excluded. It is this step in interpretation in which the true skill in interpretation resides (i.e., achieving high specificity is more challenging than high sensitivity). If this cannot be done well, too many patients are sent on to colonoscopy for false-positive CTC examination results from pseudopolyps. Young and colleagues have shown that nonradiologists can detect potential polyps with similar sensitivities to radiologists at the 8-mm threshold but at the expense of specificity (78% vs. 92.2%, respectively). This suggests that nonradiologists do not have critical cross-sectional characterization skills.
The property of polyp coating from tagging agents is an important phenomenon that has now been recognized. It positively affects detection and characterization, perhaps most importantly for flat polyps. As noted earlier (see “Bowel Preparation”), tagging agents adhere to true polyps while are washed away from the normal mucosa. This serves to draw attention to possible polyps, particularly on the 2D view, and helps increase confidence that a polyp is real (by demonstrating this property). One pitfall is not to mistake poorly tagged stool or adherent plaques of tagged stool as coated polyps (see later, “Characterization”).
Detection
Regarding detection, there had been a heated debate in the literature about the optimal method, a primary 2D approach or primary 3D approach. A primary 2D approach consists of interrogating a stack 2D dataset, typically in the transverse plane ( ). The mechanics are similar to viewing a standard CT, in which the reader scrolls interactively through a stack of images in polyp window settings (2000 W, 0 L). The colon is traced from rectum to cecum with the intent of detecting focal soft tissue projections into the colonic lumen. The difficulty of this approach is distinguishing between a focal polyp and colonic haustral fold. On a single image, a colonic fold can present as a focal polyp; it requires scrolling through to ascertain its elongated nature ( Fig. 53-6 ). Once experienced, the reader can be very effective at quickly detecting polyps by this approach. However, it requires sustained concentration, which can be somewhat tedious. The 2D search pattern is analogous to detecting pulmonary nodules on a chest CT and distinguishing them from pulmonary vessels.
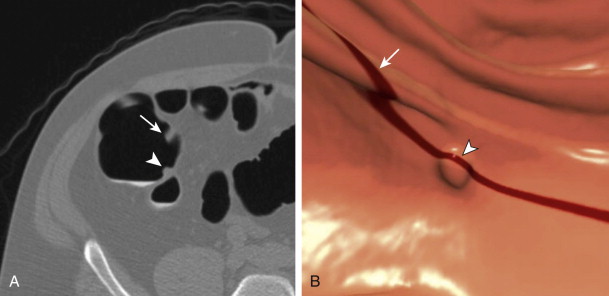
A primary 3D approach uses postprocessing of the 2D dataset into a 3D perspective. The reading mechanics are completely different and involves traveling through a tubular 3D environment, typically on a preprogrammed course (see ). When needed, the reader can then break off this fly-through to interact freely in this 3D environment and visualize an area of concern from any angle. The 3D approach holds the advantage that differentiating potential focal polyps from colonic folds is easily done from this perspective (see Fig. 53-6 ). The mental translation into a 3D structure that is needed for a primary 2D approach has been undertaken by a computer. Whereas from a 2D perspective, a haustral fold can appear polyp-like until assessed in detail, such is not the case at 3D where they are instantly recognizable as different entities. A disadvantage of this approach is that 3D relies on surface morphologic cues only as compared to the 2D approach, which uses morphologic and attenuation cues simultaneously. Thus, any retained stool, despite its tagged status, would appear as a polyp at 3D and require evaluation of the 2D source data for confirmation. Also, areas of colonic collapse or segments with residual fluid are not well evaluated with a 3D approach, and the reader may not be aware of their existence on that series. Typically, however, 3D evaluation is possible on the complementary series as fluid moves away and gas distends these areas on the second series.
The standard 3D viewing option is an endoluminal perspective. Traditionally, a fly-through consists of passage from the rectum to the cecum and then from the cecum to the rectum to allow both sides of the colonic folds to be visualized. Often, the field of view is widened at 120 degrees. In addition to the standard endoluminal view, there are alternate advanced 3D displays in use, including the filet view, unfolded cube, and band view ( Fig. 53-7 ). These increase visualization (behind folds) and thus speed of interpretation, but at the cost of increased spatial distortion.

Despite the prior controversy regarding detection approaches, there is now general consensus that a combination of 2D and 3D is required for optimal polyp detection. In practice, it has become obvious that the approaches are complementary; polyps or masses that are difficult to visualize by one perspective are easily seen by the other. For the 2D approach, there are some polyps that project off the colonic mucosa or off the end of the fold at an angle relative to the axial plane that are difficult to detect because the reader perceives the polyp as a fold or an extension of the fold ( ). In addition, polyp shape at 2D can cause difficulties in detection ( ). Both situations are not problem areas at 3D. For 3D, polyps submerged in the polyp pool can be missed ( ). In addition, underdistended areas can make the determination of lesions difficult because the walls become irregular as a result of their partially collapsed state. Focal areas of collapse related to an annular cancer with the 3D approach can be misinterpreted and missed if not evaluated with a 2D approach ( Fig. 53-8 ). By combining 2D and 3D detection, the complementary nature of the approaches allows a polyp to be seen when not evident by one perspective. In addition, the added redundancy of interacting with the imaging dataset multiple times during a combined approach further decreases the possibility of missing polyps.