■
Introduction
Certain diffuse lung diseases are associated with high- or low-attenuation lesions. High-attenuation lesions are most often due to calcium deposition from a variety of mechanisms, but can also be due to the accumulation of other radiopaque substances such as iodine (amiodarone lung toxicity) and talc (talcosis). Low-attenuation lesions are predominantly due to the deposition of lipid-containing substances. These diffuse lung diseases can encompass a large range of causes, including infectious, inflammatory, neoplastic, metabolic, occupational exposures, iatrogenic, and idiopathic processes. To make an accurate diagnosis, radiologists need to be cognizant of the morphologies and distribution patterns of these high- or low-attenuation lesions and must be able to assimilate other relevant imaging features and clinical histories. These important distinguishing characteristics, as well as common problem-solving issues and pitfalls, will be discussed in this chapter.
■
Imaging Modalities Used to Assess Diffuse Lung Disease Involving High- or Low-Attenuation Lesions
Radiographs are usually part of the initial workup for diffuse lung disease and can demonstrate large nodules, consolidation, prominent hilar and mediastinal lymphadenopathy, and calcification. Diffuse micronodules and interstitial calcification can present as reticular or reticulonodular opacities on radiographs. However, radiographs have a low sensitivity for detecting smaller lung lesions and often fail to demonstrate fine parenchymal detail and specific attenuation of lung lesions. In addition, diffuse lung disease involving calcification or lipid can mimic pulmonary edema, interstitial lung disease, or atypical infection with radiography. CT with a high-resolution CT (HRCT) technique (thin-slice, high-resolution technique) provides optimal evaluation of diffuse lung disease and is more sensitive and specific in detecting calcification and lipid. On CT, calcified lesions show attenuation similar to that of bone, ranging from 200 to >1000 Hounsfield units (HU). Lesions containing macroscopic fat have negative attenuation values, in general between −50 and −100 HU. Calcification or lipid mixed with other materials, such as soft tissue, will produce intermediate-attenuation values; in addition, volume averaging with adjacent tissue can occur, especially when thicker slices (>1–2 mm) are used for interpretation and when attenuation measurements of very small lesions are attempted. Thin-section CT can also depict the fine parenchymal detail, including patterns of small nodules, that is necessary for the diagnosis of many diffuse lung diseases involving high attenuation or lipid.
Although nuclear medicine examinations such as a technetium-99m methylene diphosphonate ( 99m Tc-MDP) bone scan and PET can play a role in the evaluation of metastatic disease, they are not the primary modalities for the characterization of diffuse lung disease involving calcification or lipid because uptake of radiotracers can occur in a wide variety of malignant and inflammatory nonmalignant diseases. Chest MRI also has not been traditionally used as a primary method of evaluating these diseases because this modality is inferior to CT in characterizing fine pulmonary parenchymal detail, small nodules, and small foci of fat or calcium.
■
Diffuse Lung Disease With Calcification or High Attenuation
Diffuse lung disease with calcification or high attenuation can present with nodules, masses, consolidation, or a combination of these. Ancillary findings in some diseases can include calcified lymphadenopathy, septal thickening, and pleural calcification. Nodules and masses can have a wide range of sizes, from tiny nodules <1 mm to masses >10 cm. Diseases can have an upper lung, lower lung, or no particular craniocaudal predominance.
Of the many diseases presenting with nodules, many can be categorized according to the distribution pattern of the calcified lesions relative to the secondary pulmonary lobule. The distribution pattern can be centrilobular, perilymphatic, or random. Centrilobular nodules are located within the central secondary pulmonary lobule and spare pleural surfaces and interlobular septa. Perilymphatic nodules are distributed along the subpleural interstitium, bronchovascular interstitium, and interlobular septa. In a random distribution, nodules are widely distributed without a particular anatomic relation to the secondary lobule; a subset is distributed along the pleural and interlobular septa.
These distribution patterns serve as a good starting point for narrowing the differential diagnosis; additional imaging and clinical features provide further guidance. Certain rare diffuse lung diseases, such as pulmonary alveolar microlithiasis and pulmonary ossification, can have unique calcification patterns that are not typical of the classic distribution pattern discussed above. The list of differential diagnoses for different distribution patterns is summarized in Box 26.1 . In the setting of solitary, sparse, multiple calcified nodules or nodules that are large enough to distort the pulmonary lobular architecture, which can be observed in healed granulomatous infection, calcified pulmonary metastasis, and hyalinizing granuloma, the distribution pattern can be difficult to determine, making other diagnostic clues such as clinical histories and other associated imaging features important in problem solving. (See Table 26.2 for a summary of the calcification pattern and associated imaging and clinical features of each disease entity that help differentiate between disease entities that share a similar imaging presentation and identify diseases where the calcification pattern alone provides very limited information.) A disease entity also can have multiple presentations on imaging, adding to the complexity of problem solving.
Calcified Nodules
Random Distribution
- ■
Healed granulomatous infection
- ■
Healed varicella pneumonia
- ■
Calcified pulmonary metastasis
Perilymphatic Distribution
- ■
Pneumoconioses
- ■
Sarcoidosis
- ■
Amyloidosis (diffuse parenchymal form)
Centrilobular Distribution
- ■
Pulmonary hemosiderosis
- ■
Metastatic pulmonary calcification
Other Patterns of “Calcification”
- ■
Pulmonary ossification
- ■
Pulmonary alveolar microlithiasis
Calcified Masses (>3 cm) or Consolidation
- ■
Progressive massive fibrosis
- ■
Amyloidosis (nodular form)
- ■
Metastatic pulmonary calcification
- ■
Hyalinizing granuloma
High-Attenuation Nodules or Masses Without Visible Calcification
- ■
Talcosis
- ■
Amiodarone lung toxicity
- ■
Pulmonary alveolar microlithiasis
■
Diseases Causing Calcified Pulmonary Nodules
These can be in a random, perilymphatic, or centrilobular distribution.
Random Distribution
Healed Granulomatous Infections
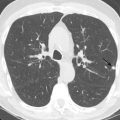
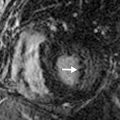
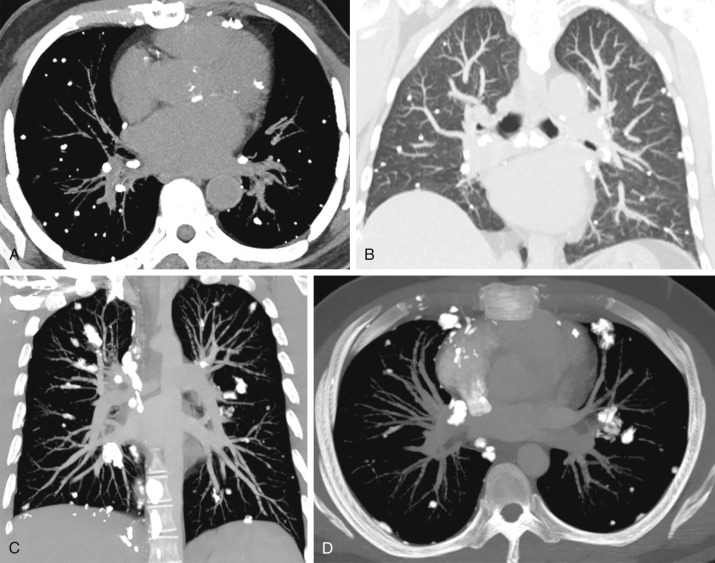
Healed granulomatous infections are common causes of solitary and multiple calcified nodules in the lung parenchyma. The nodules usually follow a random distribution or can occur in clusters ( Fig. 26.1A and B ). In the United States, histoplasmosis is the most common cause of granulomatous infection, whereas tuberculosis is the leading cause worldwide. Other rare causes include coccidioidomycosis and blastomycosis. The pulmonary manifestations of acute primary granulomatous infection vary widely and depend on the amount of exposure, the virulence of the infectious organisms, and the strength of the host’s immune system. A granuloma is an area of caseating necrosis that was once a focus of infection, later contained by the host’s cell-mediated immune response and subsequently encapsulated by fibrotic tissue. Over the course of months to years, dystrophic calcifications develop with the granulomas. Depending on the size of inoculum, the number of granulomas can vary. Calcification of the diseased lymph nodes and hepatic and splenic granulomas can also be seen with healed granulomatous disease. The imaging appearance of healed histoplasmosis and healed tuberculosis can be indistinguishable. However, multiple splenic calcifications and diffuse nodular calcification patterns are much more commonly seen in histoplasmosis; in addition, significant architectural distortion and/or bronchiectasis with asymmetric, upper lung predominance are more common findings in tuberculosis.
Healed Varicella Pneumonia
Varicella pneumonia is the one of the most common and most severe manifestations of disseminated varicella zoster virus infection in adults, with reported mortality rates of 9% to 50%. Its incidence increases with age, with a peak between the third and fifth decades of life. Some of the risk factors for developing varicella pneumonia include lymphoma, leukemia, immunocompromised status (e.g., as with chronic corticosteroid usage), pregnancy, and a history of smoking. Varicella pneumonia typically develops 3 to 5 days after the classic polymorphic rash (blisters, pustules, and crusty lesions). The predominant histologic finding for acute varicella pneumonia is diffuse alveolar damage. On radiographs, acute varicella pneumonia often presents as multiple ill-defined nodules from 5 to 10 mm, usually without associated mediastinal and hilar lymphadenopathy or pleural effusions. CT reveals multiple noncalcified, coalescent 1- to 10-mm pulmonary nodules with surrounding ground-glass opacity (halo sign) in a centrilobular pattern, with multiple patchy areas of ground-glass opacity. Typical management involves acyclovir and supportive care. Depending on the severity of disease, these pulmonary nodules may resolve within days or up to several weeks following clearing of the skin rash. Some of the nodules can become healed granulomas and gradually evolve into diffuse, calcified, small nodules (1–3 mm). Healed varicella pneumonia can mimic healed histoplasmosis. However, concurrent hepatic and splenic calcified granulomas, if present, favor histoplasmosis as the underlying cause.
Calcified Pulmonary Metastases
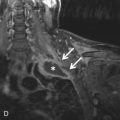
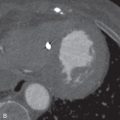
Certain malignancies that metastasize to the lungs can manifest as solitary or multiple calcified nodules on imaging. The most commonly encountered example is osteosarcoma, the most common malignant bone tumor in children and young adults (see Fig. 26.1C and D ). Up to 90% of patients with osteosarcoma have been found to have pulmonary metastases on autopsy. Tumor cells spread to the lungs hematogenously. A random but overall lower lobe–predominant pattern is observed due to relatively higher perfusion in the lower lobes. Like their primary source, metastatic pulmonary nodules can calcify by heterotopic bone formation within the tumor osteoid matrix. 99m Tc-MDP bone scan can be used to detect metastatic osteosarcoma. However, pulmonary metastatic lesions may not calcify until they grow larger, thereby limiting the sensitivity of bone scanning for detecting small soft tissue metastatic lesions. Therefore, bone scanning is usually used in conjunction with CT. Other primary sarcomas that may show calcified metastases include synovial cell sarcomas, chondrosarcomas (by bone formation within tumor cartilage), and giant cell tumor.
In addition, other nonsarcoma extrapulmonary malignancies have been shown to have calcified lung metastases, including papillary and mucinous adenocarcinoma (of the gastrointestinal tract and ovary) and thyroid medullary carcinoma. In the setting of metastatic mucinous adenocarcinoma, calcium salt can deposit in areas of mucinous degeneration (mucoid calcification), yielding partially calcified nodules with stippled, irregular, or globular eccentric calcification. In the setting of thyroid medullary carcinoma, calcification of metastatic nodules is at least in part due to psammoma body formation. It is also important to note that any treated malignancy in the lung, metastatic or primary, can calcify by dystrophic calcification.
Perilymphatic Distribution
Pneumoconioses
Pneumoconioses are irreversible lung diseases caused by the chronic inhalation of microparticles, such as crystalline silica (silicosis), coal particles (coal workers’ pneumoconiosis), beryllium particles (berylliosis), iron oxides (siderosis), tin oxides (stannosis), and barium dust (baritosis). Obtaining the occupational history is essential for the diagnosis of such diseases. Silicosis is associated with mining, sandblasting, stonecutting, quarrying, and manufacture of pottery or glass. Berylliosis is associated with the ceramic, fluorescent light bulb, nuclear weapon, and aerospace industries. Siderosis is associated with iron welding and metal polishing. Stannosis is associated with tin smelting. Baritosis is associated with the chronic inhalation of barium sulfate dusts, which are associated with the manufacture of paint, paper, textile, and numerous other items. Table 26.1 summarizes several important pneumoconioses with their typical associated occupational histories. In general, pneumoconioses have a slow disease progression. Symptoms, including productive cough and progressive dyspnea, as well as radiographic abnormalities, usually follow a latency period of about 10 to 20 years after exposure. However, latency period and disease presentation may change in the setting of high exposure. For example, accelerated silicosis, which can occur in the setting of chronic high exposure, can have a shortened latency of 5 to 10 years with a more severe disease presentation. Acute silicoproteinosis, which is another variant of silicosis caused by high exposure for months to 2 years, can present clinically and pathologically similar to alveolar proteinosis (see below for further discussion). Unlike silicosis, berylliosis has a variable latency period of 2 months to >40 years and can manifest clinically in the setting of minimal exposure. This is largely because its pathogenesis involves activation of adaptive and innate (inflammatory) immune responses.
| PNEUMOCONIOSES | OCCUPATIONAL HISTORIES |
|---|---|
| Silicosis | Mining, sandblasting, stonecutting, quarrying, construction, pottery, glass, ceramics manufacture |
| Coal workers’ pneumoconiosis | Coal mining |
| Beryllium | Ceramics, fluorescent light bulb, nuclear weapons, dental prostheses, alloy machining, automotive and aerospace industries |
| Siderosis | Iron welding and metal polishing |
| Stannosis | Tin smelting and bagging |
| Baritosis | Paint, paper, textile, leather, vinyl, rubber, linoleum, alloy manufacture |
| Talcosis | Chronic inhalation of baby powder or cosmetic talc; mining, production of paint, rubber, paper, leather, ceramics, and insecticide |
The typical radiographic findings of chronic simple silicosis and coal workers’ pneumoconioses are reticulonodular opacities; calcification may be identified within nodules. On CT, pneumoconioses usually present as numerous small nodules (<10 mm) in a perilymphatic distribution, with or without calcification ( Fig. 26.2 ). These nodules tend to be upper lobe predominant (especially the posterior portions) due to relatively poor lymphatic clearing of the microparticles from the alveoli in the upper lobes; this is a result of lower perfusion and limited respiratory excursion toward the lung apices, with resulting relatively lower lymphatic clearance. Bilateral hilar and mediastinal lymphadenopathy is common, especially in silicosis. Peripherally calcified lymphadenopathy, also known as eggshell calcification , can be seen in silicosis, coal workers’ pneumoconiosis, and sarcoidosis. In complicated pneumoconioses, the micronodules can grow and form conglomerates (>1 cm) that can gradually migrate toward the hila, a finding known as progressive massive fibrosis. When a conglomerate mass grows larger than 4 cm, it can develop central necrosis and punctate calcifications. With similar imaging findings, sarcoidosis can mimic certain pneumoconioses. However, unlike pneumoconioses, sarcoidosis can often present as an isolated lymphadenopathy without parenchymal disease. Typical management involves removal of exposure and supportive care.
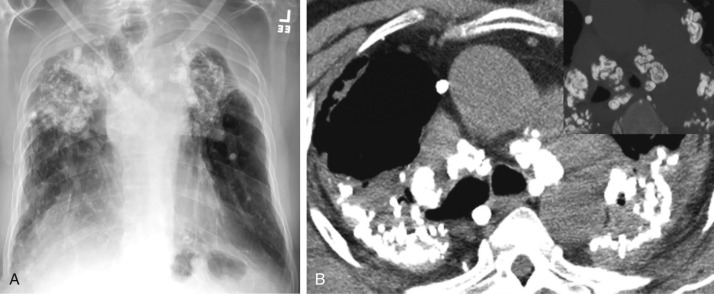
Sarcoidosis
Sarcoidosis is an idiopathic chronic inflammatory disease with noncaseating granulomas as the predominant histologic finding. It typically affects young adults with peak age of 20 to 29 years at presentation, with a slight female predominance. In the United States, the age-adjusted annual incidence among African Americans is more than threefold higher than that among whites (35.5 vs. 10.9/100,000 people). African Americans, Swedes, and Danes have the highest prevalence worldwide. Although sarcoidosis in whites tends to be subclinical, the disease presentation in African Americans tends to be more severe and can involve multiple systems, including the heart, nervous system, bone, joints, muscles, skin, eyes, and kidneys, but most commonly the lung. Common symptoms include cough, dyspnea, fatigue, night sweats, weight loss, and erythema nodosum. However, up to 50% of cases remain asymptomatic and are often found incidentally on imaging. Studies have shown that the underlying disease mechanism may be related to a variety of factors, including environmental exposures and genetic, infectious, and immunologic mechanisms.
Pulmonary sarcoidosis usually manifests radiographically as mediastinal and hilar lymphadenopathy (Scadding stage 1) and may develop punctate, amorphous, or eggshell calcification. More advanced disease (stage 2) can have parenchymal and nodal involvement. As the parenchymal disease progresses toward fibrosis, lymphadenopathy can disappear (stage 3). Parenchymal involvement of sarcoidosis can present as reticulonodular opacity with radiography.
On CT, it can present as diffuse, upper lobe–predominant, small nodules in a perilymphatic distribution, nodular septal thickening, and patchy areas of ground-glass opacities. Nodular bronchial and bronchiolar wall thickening can also cause mosaic attenuation of the lung. In complicated disease, nodules can aggregate and form masslike lesions in the upper lobes, with architectural distortion. Although most sarcoidosis cases remain stable or enter remission within a decade after diagnosis, without significant clinical consequences, approximately 20% of patients can progress to end-stage pulmonary sarcoidosis (stage 4; Fig. 26.3 ), with irreversible, upper lobe–predominant honeycombing, bullae, traction bronchiectasis, and architectural distortion. For many mild cases, symptomatic relief can be achieved by the use of nonsteroidal antiinflammatory drugs. For active pulmonary sarcoidosis that does not spontaneously resolve in 2 to 3 months, a corticosteroid is often used, despite lack of strong evidence of its efficacy. Severe symptoms often require immunosuppressive agents, such as methotrexate.
Amyloidosis
Amyloidosis has a wide spectrum of imaging presentations in the chest, often mimicking that of other disease entities. Amyloidosis is a multisystem disease in which organ dysfunction is caused by extracellular deposition of insoluble beta-pleated sheets, formed by the overexpression of a range of different proteins. Amyloidosis has a male predominance, with a mean age of 55 to 60 years at the onset of disease. Isolated pulmonary amyloidosis can occur, but up to 80% to 90% of cases of amyloidosis are thought to have systemic involvement. Systemic amyloidosis can be idiopathic (primary) or reactive (secondary) to underlying chronic inflammatory conditions, such as multiple myeloma, rheumatoid arthritis, chronic infections, and familial Mediterranean fever. The clinical presentation depends on the organ involved; the heart and kidney are usually involved in amyloidosis and can manifest as heart failure and nephrotic syndrome.
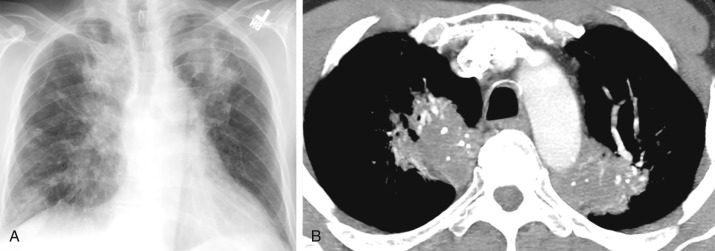
Two types of pulmonary parenchymal amyloidosis are recognized: nodular and diffuse. The nodular type is typically seen in localized amyloidosis and is usually asymptomatic; the diffuse parenchymal type is usually seen in systemic amyloidosis and carries a poor prognosis, with progressive dyspnea and a mean survival of 16 months. On imaging, the nodular type can manifest as a stable or slow-growing single node or multiple nodules or masses ( Fig. 26.4 ). Nodules range from 0.5 to 15 cm in size. Although some cases have a diffuse distribution of nodules throughout the lung parenchyma, a peripheral and lower lobe predominance occurs in others. Dystrophic calcification can develop within the nodules or masses. The pattern of calcification can be amorphous and sometimes eccentric, at times mimicking either healed granulomatous infection or malignancy. Thin-walled cysts and cavitary nodules may coexist with solid nodules. The diffuse parenchymal form of amyloidosis manifests as an interstitial opacity, interlobular septal thickening, small nodules (<1.5 cm) in a perilymphatic distribution, and, less commonly, traction bronchiectasis and honeycombing. Confluent nodules and consolidation can be seen in a subset of cases.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree