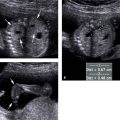
Figure 4.1.1
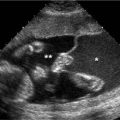
Hydrocephalus. Axial view of fetal head at 25 weeks gestation demonstrating dilated lateral ventricle, with + calipers measuring the width of the atrium of the lateral ventricle as 12.8 mm. The calipers are aligned perpendicular to the axis of the ventricle.
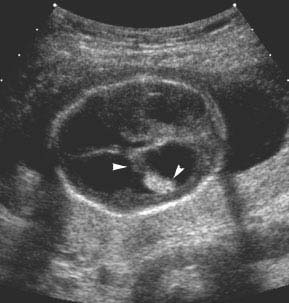
Figure 4.1.2
Hydrocephalus with dangling choroid plexus. Axial view of a 19-week fetus with hydrocephalus, demonstrating choroid plexus (arrowheads) dangling from its medial attachment toward the lateral wall of the ventricle.
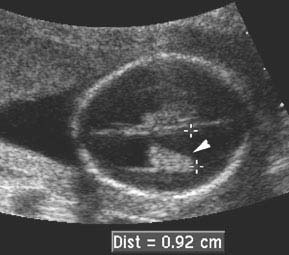
Figure 4.1.3
Hydrocephalus in a 16-week fetus. Dilated ventricle (+ calipers) with dangling choroid plexus (arrowhead). Measurement is less than 10 mm (9.2 mm) despite the presence of hydrocephalus, because of the early gestational age.
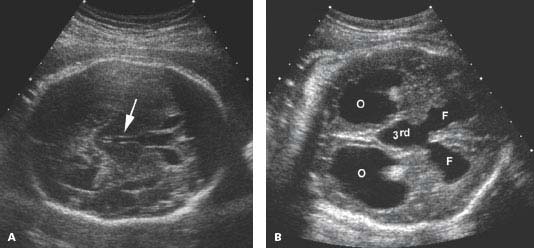
 Figure 4.1.4
Figure 4.1.4
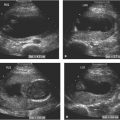
Dilated third ventricle with hydrocephalus. A: Axial image of head in a fetus with mild hydrocephalus showing mildly dilated third ventricle (arrow) between the thalami. B: Oblique view of head demonstrating dilated third ventricle communicating with dilated frontal horns (F). The dilated occipital horns of the lateral ventricles (O) are also visible.
4.2. Aqueductal Stenosis
Description and Clinical Features
Aqueductal stenosis is obstruction of the cerebral ventricular system at the aqueduct of Sylvius, which lies between the third and fourth ventricles. Aqueductal stenosis leads to hydrocephalus, with dilation of the lateral and third ventricles. The fourth ventricle and posterior fossa are normal. Aqueductal stenosis is sometimes the result of an X-linked recessive genetic trait and, therefore, is more commonly found in males than in females. Other causes of aqueductal stenosis include in utero infections (e.g., toxoplasmosis, cytomegalovirus, and syphilis), or teratogen exposure.
Sonography
With aqueductal stenosis, both lateral ventricles and the third ventricle are dilated, while the posterior fossa and fourth ventricle are normal (Figures 4.2.1 and 4.2.2).
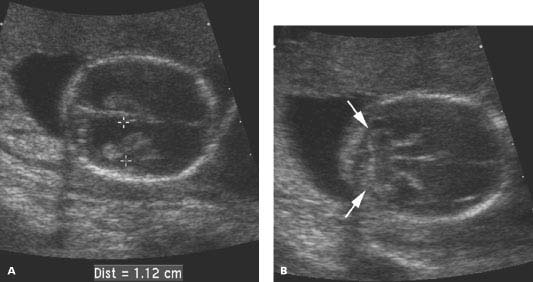
Figure 4.2.1
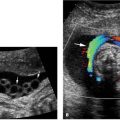
Aqueductal stenosis at 17 weeks. Axial views of fetal head demonstrating (A) a dilated lateral ventricle (calipers) and (B) a normal posterior fossa containing the cerebellum (arrows).
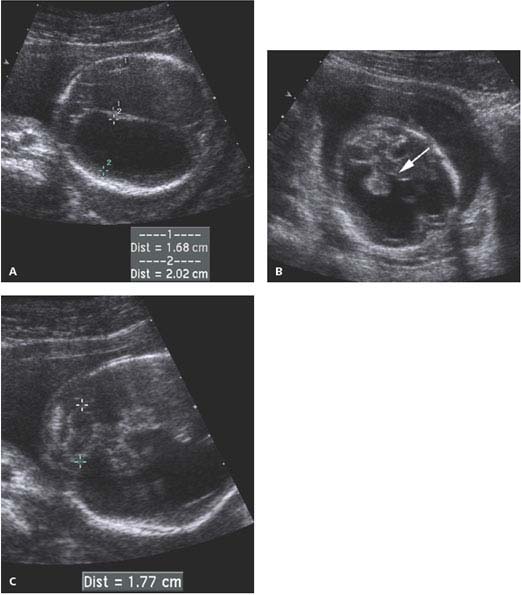
Figure 4.2.2
Aqueductal stenosis at 19 weeks. A: Axial image of head showing markedly dilated lateral ventricles (calipers). B: Oblique view demonstrating dilated third ventricle (arrow) communicating with dilated lateral ventricles bilaterally. C: The posterior fossa containing the cerebellum (calipers) is normal.
4.3. Dandy-Walker Malformation
Description and Clinical Features
The Dandy-Walker malformation is characterized by a posterior fossa cyst that communicates with the fourth ventricle, agenesis or hypoplasia of the cerebellar vermis, and hydrocephalus. The posterior fossa cyst, or Dandy-Walker cyst, is a fluid collection that extends from the fourth ventricle between the cerebellar hemispheres to the cisterna magna. The cerebellar hemispheres are separated by the fluid of the Dandy-Walker cyst, and they are often abnormally formed. The degree of hydrocephalus is variable.
Dandy-Walker malformations are associated with various genetic syndromes and chromosomal abnormalities. They can also result from in utero infection.
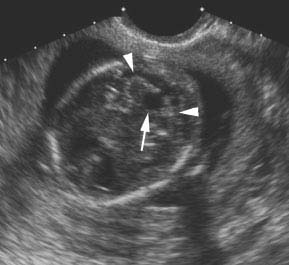
 Figure 4.3.1
Figure 4.3.1
Dandy-Walker malformation. Angled coronal view through head demonstrating a keyhole-shaped defect (arrow) between the cerebellar hemispheres (arrowheads) due to absence of the cerebellar vermis, permitting communication between the fourth ventricle and the cisterna magna.
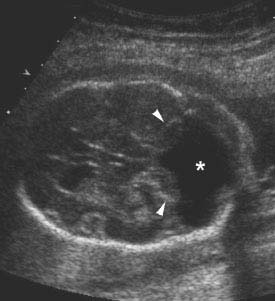
Figure 4.3.2
Dandy-Walker malformation with large posterior fossa cyst and flattened cerebellar hemispheres. Axial image of posterior fossa demonstrating absence of the cerebellar vermis, as well as splaying and flattening of the cerebellar hemispheres (arrowheads) by a large fluid-filled space (*) connecting the fourth ventricle to the cisterna magna.
Sonography
When a Dandy-Walker malformation is present, fluid is seen between the cerebellar hemispheres and the cerebellar vermis is absent or hypoplastic (Figure 4.3.1). The Dandy-Walker cyst often has a characteristic keyhole shape between the cerebellar hemispheres. The posterior aspect of the cyst may be quite large, and the cerebellar hemispheres are sometimes flattened (Figure 4.3.2) rather than having their normally rounded shape. Hydrocephalus (Figure 4.3.3) often accompanies the posterior fossa abnormalities.
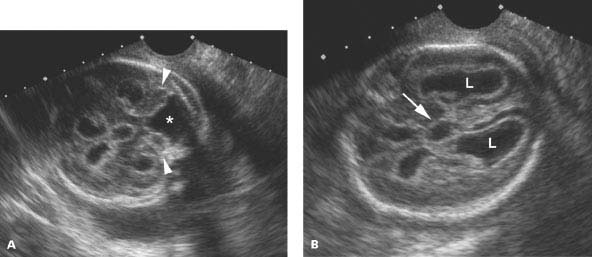
Figure 4.3.3
Dandy-Walker malformation with hydrocephalus. A: Angled image of head demonstrating flattened cerebellar hemispheres (arrowheads) splayed by large posterior fossa cyst (*). B: Axial image above the level of (A) demonstrating dilated lateral ventricles (L) and third ventricle (arrow).
4.4. Anencephaly
Description and Clinical Features
Anencephaly is a neural tube defect involving the fetal head, characterized by absence of the cranium. Dystrophic brain tissue may form in the empty shell behind the face, but this tissue tends to atrophy as pregnancy progresses. The face, from the orbits down, is usually normally formed. Anencephaly makes up approximately 45% of all neural tube defects.
This severe anomaly is sometimes diagnosed during the latter part of the first trimester, such as at the time the fetus is scanned for measurement of the nuchal translucency. With anencephaly, very high maternal serum α-fetoprotein levels are typically present at the time of the serum screen in the second trimester. As a result, many cases not identified in the first trimester are detected in the second trimester because of widespread maternal serum screening, as well as the frequent use of ultrasound for second trimester anatomy surveys.
Polyhydramnios is commonly present, likely because fetal swallowing is impaired. The prognosis is dismal, with virtually all fetuses dying at birth.
Sonography
Absence of the fetal cranium is the hallmark of anencephaly. During the first trimester, dystrophic brain tissue is commonly seen as amorphous tissue protruding from the skull base and floating above the face (Figures 4.4.1 and 4.4.2). By the second trimester, dystrophic tissue is less often present in the expected region of the cranial vault. In such cases, the lower face will be seen, with no skull or tissue above the orbits anteriorly (Figure 4.4.3) or above the cervical spine posteriorly. Polyhydramnios may be present.
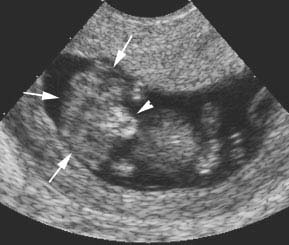
 Figure 4.4.1
Figure 4.4.1
Anencephaly with dystrophic brain tissue in region of absent cranium. Eleven-week fetus with anencephaly demonstrating amorphous mass of brain tissue (arrows) where the cranium should be. The bones of the lower face (arrowhead) are visible beneath the tissue.
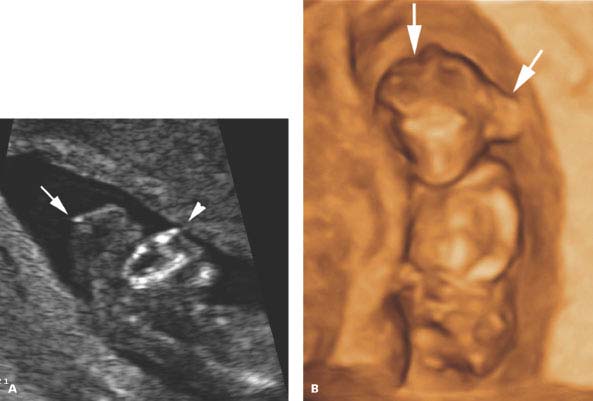
Figure 4.4.2
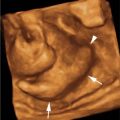
Anencephaly. A: Sagittal image of face and head of 13-week fetus demonstrating absence of the cranium. There is dystrophic tissue (arrow) above the face (arrowhead). B: Three-dimensional (3D) image demonstrating the amorphous mass of dystrophic tissue (arrows) protruding from the skull base behind and above the face.
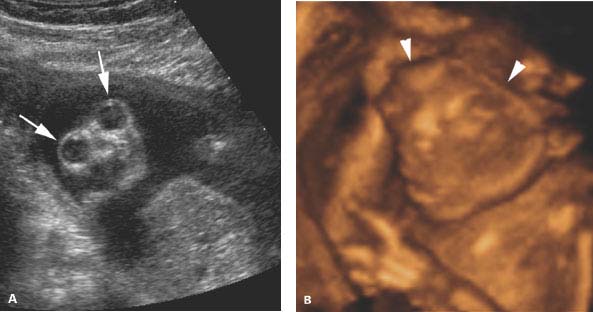
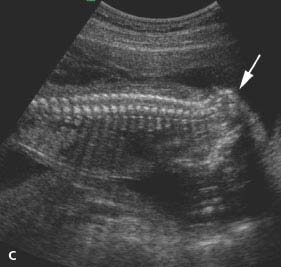
Figure 4.4.3
Anencephaly in second trimester. A: Coronal image of fetal face demonstrating absence of the forehead and cranium above orbits (arrows). The lower face is normally formed. B: Three-dimensional (3D) image of anencephalic fetus with absence of the cranium above the orbits (arrowheads) and a normal lower face below. C: Longitudinal view of cervical and thoracic spine showing absence of the cranium above the cervical spine (arrow).
4.5. Encephalocele
Description and Clinical Features
Encephalocele is characterized by a defect in the cranium through which the intracranial contents herniate outside the skull. Occipital encephaloceles are a form of neural tube defect, comprising approximately 5% of neural tube defects. Encephaloceles can also result from amniotic band syndrome and may be found with Meckel-Gruber syndrome, an autosomal recessive genetic syndrome. The findings with Meckel-Gruber syndrome include encephalocele, polycystic kidneys, polydactyly, cleft palate, cardiac anomalies, and liver cysts. Severe oligohydramnios is usually present with Meckel-Gruber syndrome, due to absent or decreased urine production from the polycystic kidneys.
Like other neural tube defects, maternal serum α-fetoprotein levels are usually elevated with encephaloceles. Occasionally, the defect is closed, covered by the scalp or skin, in which case the maternal serum α-fetoprotein level is likely to be normal.
The majority of encephaloceles are located in the midline, most commonly posterior involving the occiput, less commonly parietal or frontal. Encephaloceles that result from amniotic band syndrome may involve any part of the skull and are often not in the midline.
The herniated encephalocele sac may contain dystrophic brain tissue or it may contain only meninges and fluid. Within the head, there may be associated hydrocephalus, and, in some cases, the “lemon” sign, characterized by flattening or concavity of the frontal bones, may be seen during the second trimester.
The prognosis is variable, depending on the location of the encephalocele and the amount of brain tissue herniated into the encephalocele sac.
Sonography
The cranial defect of an encephalocele appears as an interruption in the bony skull with intracranial contents herniating through the defect (Figure 4.5.1). The herniated sac is usually rounded and contains tissue as well as fluid. With an anterior encephalocele, a soft-tissue mass will be seen protruding anteriorly through a defect in the frontal bones between the orbits (Figure 4.5.2). Hypertelorism, or widening of the normal distance between the orbits, may be present. On a sagittal midline view of the fetal face, the encephalocele may be seen as a protrusion of soft tissue between the forehead and the tip of the nose (Figure 4.5.2).
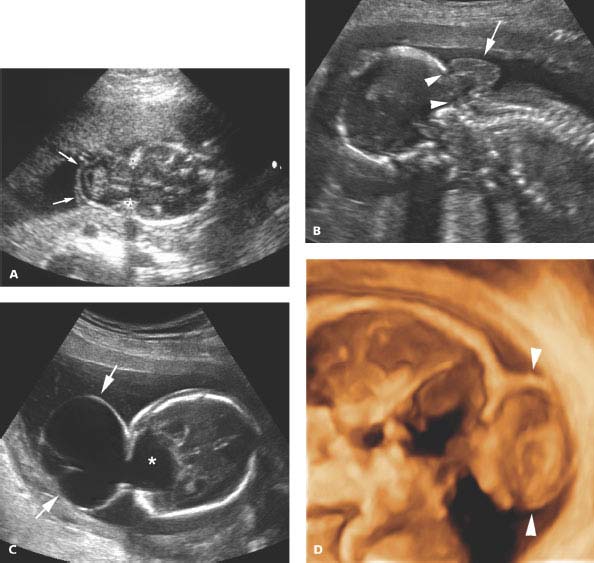
Figure 4.5.1
Occipital encephalocele. A: Calipers mark a bony defect in the occiput through which intracranial contents herniate outside the skull into the encephalocele sac (arrows). B: Sagittal image of a different fetus demonstrating the encephalocele sac (arrow) herniated through a defect in the occiput (arrowheads) above the cervical spine. C: Occipital encephalocele (arrows) filled primarily with fluid, communicating with fluid (*) within the posterior aspect of the head. D: Three-dimensional (3D) image through middle of encephalocele and head. The outer contour of the encephalocele is delineated (arrowheads). The encephalocele and posterior aspect of the head appear empty with this type of 3D imaging, because they are filled with fluid.
Encephaloceles seen with Meckel-Gruber syndrome are often difficult to visualize because of severe oligohydramnios (Figure 4.5.3). In such cases, the fetal kidneys will be enlarged and appear abnormal, either abnormally echogenic or filled with multiple cysts.
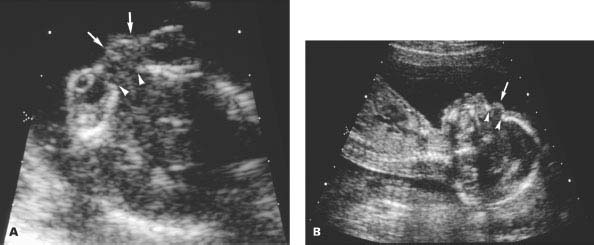
Figure 4.5.2
Anterior encephalocele. A: Axial image through orbits demonstrating defect in nasofrontal bone (arrowheads) with small encephalocele sac (arrows) protruding between the orbits anteriorly. B: Sagittal image of facial profile showing nasofrontal bony defect (arrowheads) and protruding anterior encephalocele sac (arrow).
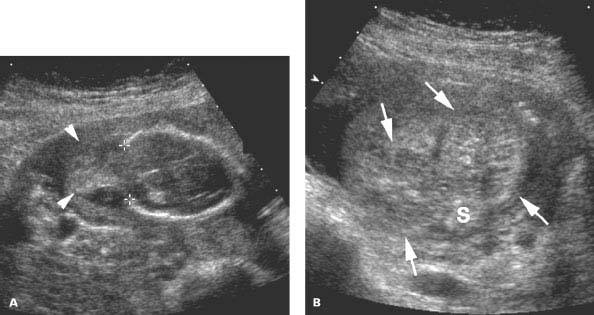
Figure 4.5.3
Meckel-Gruber syndrome. A: Image of fetal head demonstrating large occipital defect (calipers) with herniated brain tissue in the encephalocele sac (arrowheads) outside the cranium. The outer contour of the sac is difficult to see due to oligohydramnios. B: Transverse image of bilateral enlarged polycystic kidneys (arrows) on either side of the spine (S), another characteristic of this autosomal recessive syndrome.
4.6. Agenesis of the Corpus Callosum
Description and Clinical Features
The corpus callosum is a band of neural tissue that connects the right and left cerebral hemispheres. Agenesis of the corpus callosum results from failure of formation of part or all of the corpus callosum. Because the anterior portion of the corpus callosum develops before the posterior portion, partial agenesis of the corpus callosum typically affects the posterior aspect.
Agenesis of the corpus callosum is often associated with intracranial and extracranial anomalies, as well as various syndromes and chromosomal abnormalities. Other central nervous system anomalies are present in about 85% of cases, including such anomalies as Dandy Walker malformation and interhemispheric cysts.
Sonography
The most common sonographic finding with agenesis of the corpus callosum is an abnormal configuration of the lateral ventricles. Instead of their normal convergence anteriorly, the lateral ventricles are aligned parallel to the falx and displaced laterally. The occipital horns are typically disproportionately dilated, a finding termed colpocephaly (Figure 4.6.1). This latter finding may not be evident until after 20 weeks of gestation. In addition to changes in the lateral ventricles, the third ventricle is elevated and often mildly dilated (Figure 4.6.2). Cerebral sulci can be seen radiating from the roof of the third ventricle outward (Figure 4.6.3).
The cavum septum pellucidum is absent with agenesis of the corpus callosum; thus, sonographic visualization of a cavum septum pellucidum excludes the diagnosis of complete agenesis. Because the cavum septum pellucidum may be present with partial agenesis of the corpus callosum, partial agenesis is more often missed in utero than is complete agenesis.
Careful sonographic assessment for other fetal anomalies, especially of the brain, is warranted when the diagnosis of agenesis of the corpus callosum is made, because of its high association with other malformations.
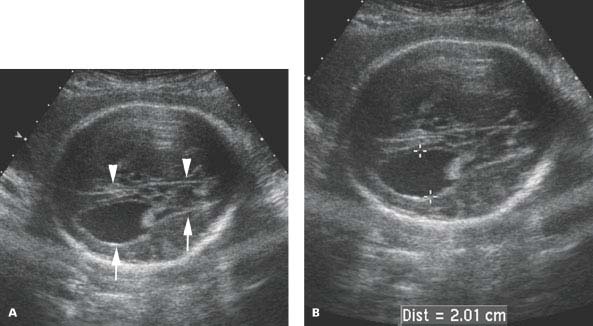
Figure 4.6.1
Agenesis of the corpus callosum with parallel alignment of the lateral ventricles and colpocephaly. Axial images of fetal head (A) demonstrating lateral ventricle (arrows) oriented in parallel to the falx (arrowheads) and (B) dilated occipital horn, measuring 2.01 cm (calipers), representing colpocephaly.
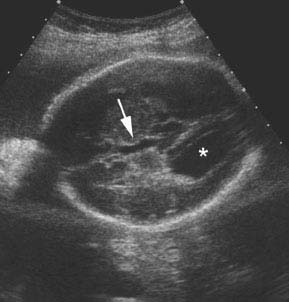
Figure 4.6.2
Agenesis of the corpus callosum with elevation of the third ventricle. Axial image of head demonstrating third ventricle (arrow) dilated and elevated such that it is seen at the level of the lateral ventricle, which has colpocephaly (*).
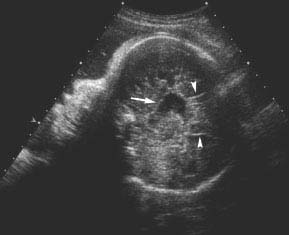
Figure 4.6.3
Agenesis of the corpus callosum with sulci radiating from the roof of the elevated third ventricle. Sagittal midline image demonstrating sulci (arrowheads) radiating from roof of the third ventricle (arrow).
4.7. Holoprosencephaly
Description and Clinical Features
Holoprosencephaly is a developmental anomaly of the brain in which the prosencephalon (forebrain) fails to cleave normally into two cerebral hemispheres. This results in complete or partial fusion of the cerebral hemispheres and communication of the lateral ventricles across the midline. The falx is underdeveloped or absent. The corpus callosum is usually absent. The thalami may be partially or completely fused.
Holoprosencephaly can have varying degrees of severity, depending on the extent of failure of cleavage of the prosencephalon. Alobar holoprosencephaly is the most severe form, characterized by complete failure of cleavage, fusion of the cerebral hemispheres, complete absence of the falx, and a large central cerebral ventricle or monoventricle. The thalami are usually fused. Semilobar holoprosencephaly has partial cleavage of the prosencephalon, with a rudimentary falx indenting the fused cerebral hemispheres in the midline. The ventricles have a wide connection across the midline, and the thalami may be partially fused. With lobar holoprosencephaly, the least severe form, the falx is present although incomplete; the cerebral hemispheres are partially separated and partially fused, and the ventricles have a narrow communication across the midline.
Holoprosencephaly is commonly associated with an abnormal karyotype, often trisomy 13, and with fetal anomalies, particularly midline facial clefts, hypotelorism, and a proboscis.
Sonography
With holoprosencephaly, the configuration of the cerebral ventricles is abnormal. In particular, the ventricles communicate across the midline and are dilated. The third ventricle is typically absent. The falx is absent or rudimentary, and the cerebral hemispheres are fused across the midline. The thalami are partially or completely fused (Figures 4.7.1 and 4.7.2). With alobar holoprosencephaly the fused ventricle is large and centrally located, and the thalami are usually completely fused (Figure 4.7.3), whereas with semilobar holoprosencephaly, the ventricles are smaller and have a narrower communication across the midline (Figure 4.7.4). When holoprosencephaly is diagnosed, the face should be assessed for midline anomalies.
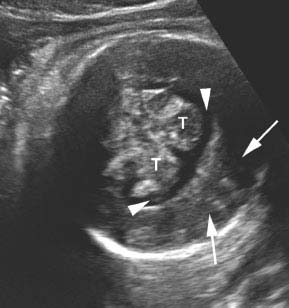
Figure 4.7.1
Holoprosencephaly. Coronal image of head demonstrating communication of lateral ventricles across the midline to form a monoventricle (arrowheads). The cerebral hemispheres (arrows) are also fused across the midline and the falx is absent. The thalami (T) are partially fused.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree