CHAPTER 12 Hepatic, splenic, and portal vascular systems
Arteriography and venography
Techniques for celiac and superior mesenteric arteriography are described in Chapter 11. Splenic or common hepatic arteriography is performed with a variety of catheters, including cobra and sidewinder shapes. Steerable, hydrophilic guidewires simplify catheter placement. Alternatively, high-flow coaxial microcatheters are used to select the main splenic or hepatic arteries and their branches.
The splenic, superior mesenteric, and portal veins are visualized on the late phases of celiac or superior mesenteric arteriography (“indirect portography”). Direct splenoportography is rarely required in the evaluation of patients with portal hypertension.1 Iodinated contrast or CO2 (15 to 20 cc) is injected through a micropuncture catheter inserted into the substance of the spleen with ultrasound guidance. The tract is embolized with Gelfoam as the catheter is withdrawn.2,3
Anatomy
Development
The liver bud develops between the pericardial cavity and the stalk of the primitive yolk sac.4 Liver cords insinuate between tributaries of the vitelline and umbilical veins to form the hepatic sinusoids. Branches of the right vitelline veins around the duodenum develop into the central portal veins.5 The right umbilical vein involutes, and the left umbilical vein becomes the primary inflow vessel to the liver. The hepatic venous outflow is directed toward the upper portion of the right vitelline vein, which ultimately forms the hepatic veins and the intrahepatic portion of the IVC. The ductus venosus connects the left umbilical vein (portal venous inflow) to the right vitelline vein (hepatic outflow). Shortly after birth, the ductus venosus and left umbilical vein close and form the ligamentum venosum and ligamentum teres, respectively.
Normal anatomy
Liver
With the advent of living-related split-liver donor transplants, more ambitious surgical techniques for segmental hepatic resection, and transcatheter methods for treatment of liver tumors, a detailed understanding of the normal, variant, and collateral hepatic circulations is critical for the vascular interventionalist. For preoperative planning, high-quality computed tomography (CT) or magnetic resonance (MR) arteriography and venography are both extremely accurate.6–9 For transarterial therapy, selective digital angiography is required, including celiac and superior mesenteric arteriography, right and left hepatic arteriography, and often more subselective catheterization.10,11
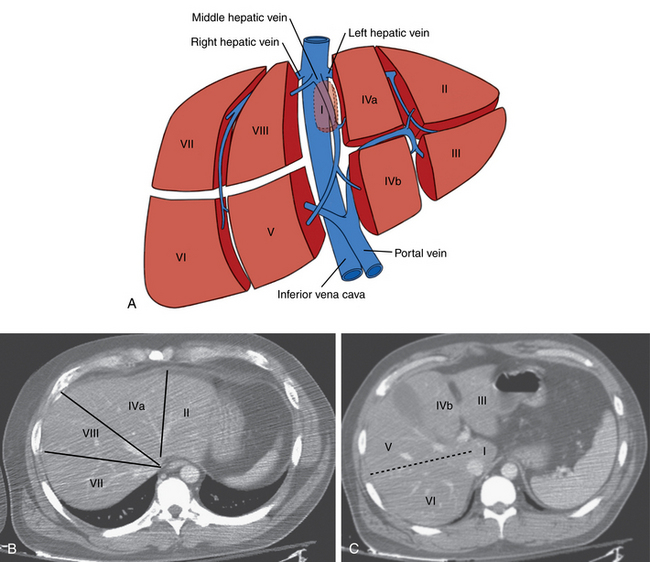
The liver is divided into right and left lobes separated by the major fissure. The right lobe has anterior and posterior segments; the left lobe has medial and lateral segments. The caudate lobe is anatomically distinct from the right and left lobes. By convention, segmental anatomy is based on the original system of Couinaud demarcated by the three main hepatic veins and a transverse plane at the level of the portal vein bifurcation12,13 (Fig. 12-1). However, the relationship between these landmarks identified on cross-sectional imaging and the true anatomic segmental anatomy is only approximate.
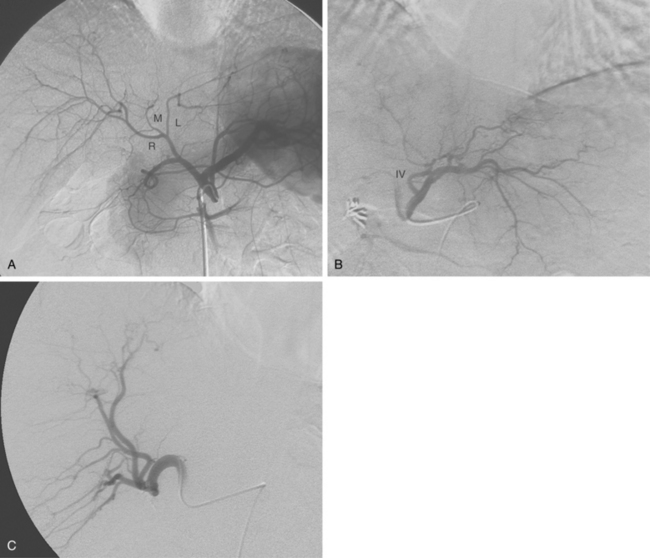
The common hepatic artery arises from the celiac artery (Fig. 12-2). After giving off the gastroduodenal artery, it becomes the proper hepatic artery. This vessel enters the porta hepatis and divides into the right hepatic artery (RHA) and left hepatic artery (LHA), which feed their respective lobes. The RHA supplies segments V to VIII (and sometimes segment I, caudate lobe). The LHA supplies segments II, III, IVa, and IVb. The inconsistent middle hepatic artery, which, if present, supplies segments IVa and IVb, usually originates from the right hepatic artery or forms a true trifurcation. Variations in hepatic arterial anatomy are common (see later discussion). Although the hepatic arteries are considered end arteries, intrahepatic and extrahepatic anastomoses do exist. The origins of important branches are outlined in Table 12-1.
Table 12-1 Important Branches of the Hepatic Arteries
| Vessel | Typical and Atypical Origins |
|---|---|
| Right gastric artery | |
| Cystic artery | |
| Supraduodenal artery | GDA, CHA, LHA, RHA, cystic |
| Dorsal pancreatic artery | |
| Falciform artery | MHA, LHA |
CHA, common hepatic artery; GDA, gastroduodenal artery; LGA, left gastric artery; LHA, left hepatic artery; MHA, middle hepatic artery; PHA, proper hepatic artery; RHA, right hepatic artery; SA, splenic artery; SMA, superior mesenteric artery.
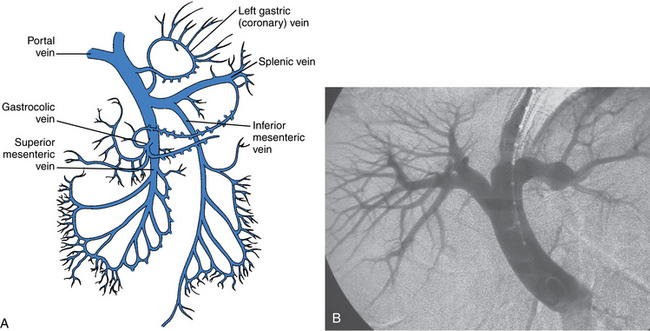
The portal vein (PV) is formed by the confluence of the superior mesenteric vein (SMV) and splenic vein14 (Fig. 12-3). It is valveless. Normal main portal vein pressure is about 3 to 5 mm Hg. The SMV has numerous jejunal, ileal, and colonic tributaries. The inferior mesenteric vein (IMV) joins the splenic vein or the SMV.15 The right gastroepiploic, pancreaticoduodenal, and right colonic veins often merge into a common gastrocolic trunk that drains into the right side of the SMV near its junction with the PV. The right and left gastric (coronary) veins join the superior surface of the main portal or central splenic vein. Multiple coronary veins often exist.
The portal vein runs anterior to the IVC and posterior to the head of the pancreas before entering the liver. The PV bifurcation is outside the liver capsule in about half of the population.16 The right and left PVs and their branches follow the hepatic artery into the liver. The left PV supplies segments I to IV.17 The right anterior PV supplies segments V and VIII; the right posterior PV supplies segments VI and VII. The caudate lobe is usually fed by branches of the left PV. The remnant of the umbilical vein (ligamentum teres) is connected to the left PV. A patent paraumbilical vein arising from the left PV is sometimes seen in patients with portal hypertension.
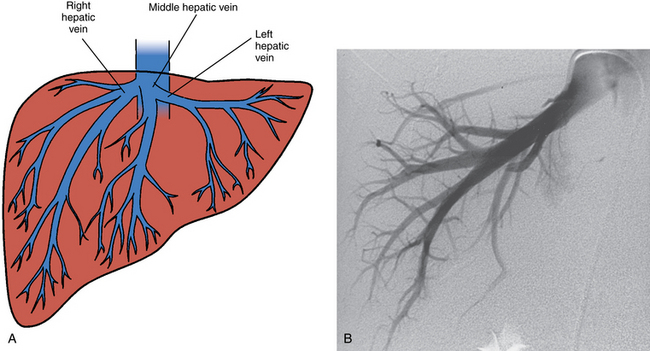
The liver is drained by the hepatic veins. The right and left hepatic veins run between the segments of the right and left lobes of the liver, and the middle hepatic vein lies in the main lobar fissure (Fig. 12-4). These vessels converge into the IVC within several centimeters of the diaphragm. The right hepatic vein (draining segments VI and VII) joins the right posterolateral surface of the IVC. The middle hepatic vein (draining segments V and VIII) and left hepatic vein (draining segments II and III) confluence enters the anteromedial surface of the IVC. Segments IVa and IVb are drained by the left or middle hepatic vein. The caudate lobe empties independently into the intrahepatic IVC.
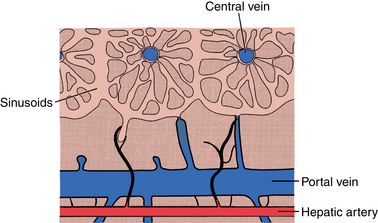
The liver parenchyma is composed of hepatic lobules, which contain the hepatocytes and sinusoidal spaces that form the functional units of the liver18 (Fig. 12-5). Neighboring lobules are organized into acini. Hepatic arterial, portal venous, and biliary duct branches follow the borders of the lobules. Hepatic arterioles feed the sinusoids directly and through communications with portal venules that perforate the lobules. Normally, blood flows freely between acini. Central veins at the core of each lobule drain the sinusoids. These venules coalesce into the hepatic veins.
Spleen
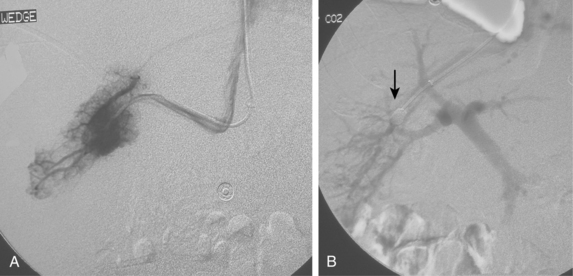
The splenic artery supplies the spleen and portions of the pancreas and stomach19 (Fig. 12-6). It follows the superior edge of the pancreas along with the splenic vein. With advancing age, the splenic artery can become extremely tortuous. Near the splenic hilum, the artery usually divides into superior and inferior branches. Superior and inferior polar arteries often arise from the midsplenic artery and supply their respective splenic segments. The left gastroepiploic artery originates from the distal inferior polar artery and then courses along the greater curvature of the stomach. Numerous short gastric branches feed the fundus of the stomach. The splenic artery also has numerous branches to the body and tail of the pancreas. The largest of these vessels are the dorsal pancreatic artery (which may originate on the celiac trunk) and the pancreatica magna artery (see Fig. 12-6).
The splenic vein lies behind the upper border of the pancreas below the splenic artery (see Fig. 12-6B). Its tributaries include the short gastric, left gastroepiploic, pancreatic, and inferior mesenteric veins (see Fig. 12-3).
Variant anatomy
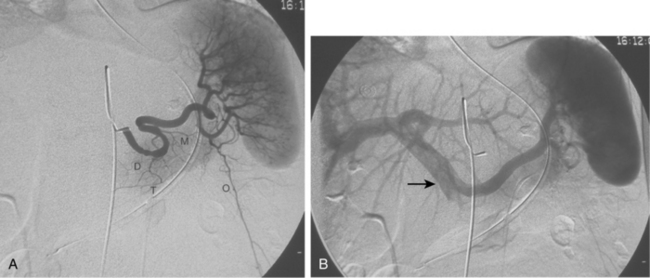
Anomalies of hepatic artery origin and number are common10,20–23 (Table 12-2). The most frequent variants are accessory and replaced hepatic arteries, such as the right hepatic from the superior mesenteric artery (SMA), the left hepatic from the left gastric, and the common hepatic from the SMA (Fig. 12-7; see also Figs. 11-7 through 11-9). Accessory hepatic arteries are those in which a portion of the affected lobe is supplied by a vessel with an aberrant origin. Replaced hepatic arteries are those in which the entire lobe is supplied by a vessel with an aberrant origin. Accessory hepatic arteries supply isolated hepatic segments and are believed by most authorities not to be redundant arteries.24
Table 12-2 Variant Hepatic Arterial Anatomy
| Type | Michels (%) | Recent Series (%) |
|---|---|---|
| I: Classic Anatomy | 55 | 58-79 |
| II: Replaced LHA | 10 | 3-12 |
| III: Replaced RHA | 11 | 6-15 |
| IV: Replaced RHA and LHA | 1 | 1-2 |
| V: Accessory LHA from LGA | 8 | 3-11 |
| VI: Accessory RHA from SMA | 7 | 3-12 |
| VII: Accessory RHA and LHA | 1 | 0-1 |
| VIII: Accessory RHA/LHA replaced LHA or RHA | 2 | 1-3 |
| IX: Replaced CHA to SMA | 4.5 | 1-2 |
| X: Replaced CHA to LGA | 0.5 | 0 |
| Double hepatic artery | 1-4 | |
| Triple hepatic artery | 0-7 | |
| Separate CHA origin from aorta | 0.4-2 | |
| Replaced PHA to SMA, GDA origin from aorta | 0.3 | |
| Other | 1.5-4 |
CHA, common hepatic artery; GDA, gastroduodenal artery; LGA, left gastric artery; LHA, left hepatic artery; PHA, proper hepatic artery; RHA, right hepatic artery; SMA, superior mesenteric artery.
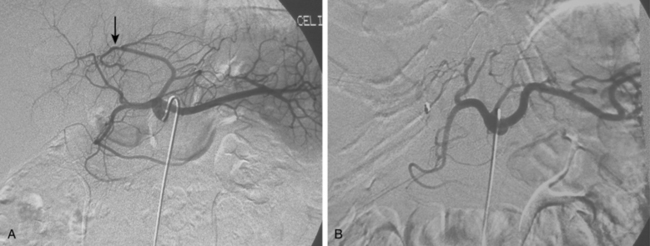
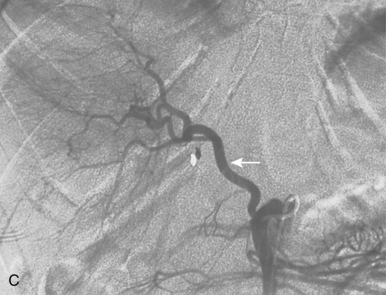
Figure 12-7 Variant hepatic arterial anatomy. A, Left hepatic artery (black arrow) replaced to the left gastric artery. B and C, Right hepatic artery (white arrow) replaced to the superior mesenteric artery.
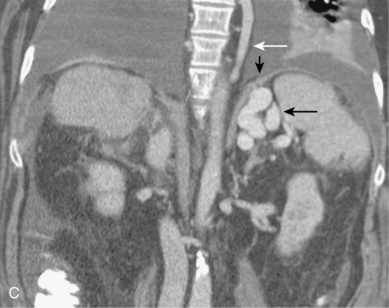
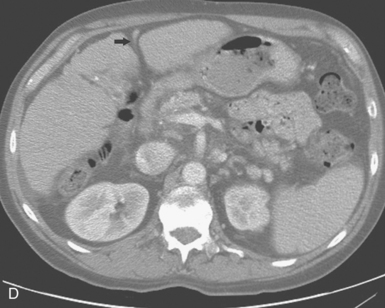
Rarely, the hepatic or splenic artery originates directly from the aorta (see Figs. 11-11 and 11-12). An accessory left gastric artery may arise from the proximal splenic artery. Important organ anomalies include an accessory spleen (usually located in the tail of the pancreas), asplenia, polysplenia, and the ectopic or “wandering” spleen.25
Classic PV anatomy is found in 65% to 75% of the population. Surgically significant variants involve trifurcation of the main PV (type 2, 9% to 16%), origin of the right posterior branch from the main PV (type 3 or “Z type,” 8% to 13%), and separate segment VI or VII branches from the right portal vein (types 4 and 5, 7%).17,26
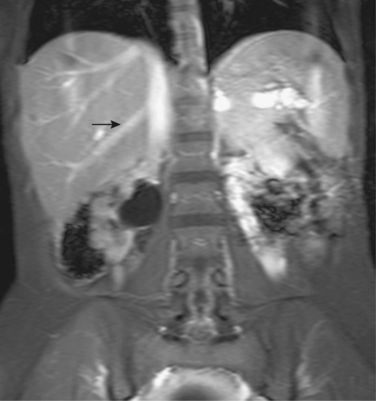
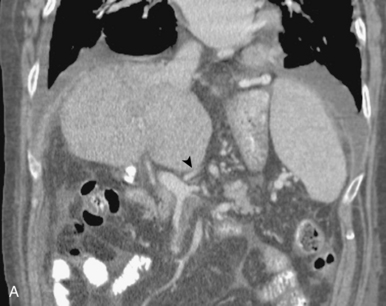
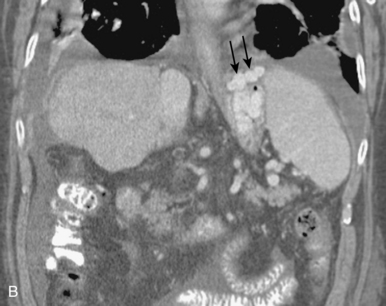
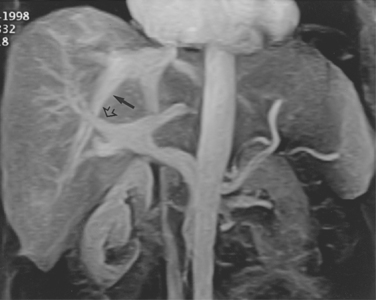
Accessory right hepatic veins are found in 25% or more of the population (Fig. 12-8). In about 3% of individuals, an inferior right hepatic vein (entering the IVC well below the diaphragm) is the dominant venous drainage for the right lobe.16
Major disorders
Cirrhosis and portal hypertension (online case 68)
Etiology
Cirrhosis is a progressive liver disease characterized by generalized necrosis, regeneration, and widespread fibrosis.27 Initially, inflammation or steatosis predominates. Fibrotic tissue then infiltrates the sinusoidal spaces and obstructs central veins while preserving portal venules. Masses (either micronodular or macronodular) begin to form, including regenerative, dysplastic, and malignant lesions. As the vascular resistance in the liver increases, PV pressure rises, but flow is still directed into the liver (hepatopetal). An imbalance in the relative activity of vasodilators (e.g., nitric oxide) and vasoconstrictors (e.g., endothelin-1) is responsible for the disturbances in hepatic, splanchnic, and peripheral hemodynamics that follow.28,29 With progression of cirrhosis, systemic and splanchnic vasodilation occurs, leading to increased cardiac output (a hyperdynamic circulatory state) and humorally mediated renal and hepatic vasoconstriction, which further impedes liver blood flow.30,31 Extrahepatic portal flow increases while intrahepatic portal flow decreases; in a compensatory fashion, hepatic arterial flow is augmented.
Although cirrhosis is by far the most frequent cause of portal hypertension, many other diseases can produce similar physiologic effects. Portal hypertension is traditionally classified by the site of obstruction relative to the hepatic sinusoids32 (Boxes 12-1 through 12-5). Some diseases affect one level and then extend to others. Disorders that cause elevated portal pressures proximal to or beyond the hepatic sinusoids are considered later in this chapter.
Worldwide, hepatitis B and C infections are the leading cause of liver cirrhosis.33Alcoholic cirrhosis is the other common form of the disease in the United States and elsewhere. It evolves from an initial stage of fatty liver to sinusoidal scarring, formation of regenerative nodules (i.e., micronodular cirrhosis), and finally widespread fibrosis with liver shrinkage. Along with diabetes and metabolic syndrome, one consequence of the epidemic of obesity is the rapidly growing incidence of nonalcoholic fatty liver disease (NAFLD), including the particularly aggressive form of nonalcoholic steatohepatitis (NASH). These infiltrative conditions are now responsible for a significant fraction of cases of liver cirrhosis.34,35
Primary biliary cirrhosis is a cholestatic liver disease of immunologic origin that results in diffuse bile duct obstruction.36 It is typically seen in middle-aged women. In patients with chronic bile duct obstruction, portal fibrosis rather than diffuse cirrhosis is the cause for portal hypertension. Hepatic schistosomiasis, which is endemic in Africa and parts of Asia, causes infiltration of portal venules and periportal spaces, leading to presinusoidal obstruction.37Congenital hepatic fibrosis presents in late childhood with features of portal hypertension but normal liver function. Idiopathic portal hypertension and noncirrhotic portal fibrosis are rare conditions in which PV pressure is elevated without underlying liver disease.38 Destruction of intrahepatic portal radicles, portal fibrosis, and liver atrophy are characteristic. Cryptogenic cirrhosis encompasses all cases without an identifiable etiology, although many of these patients may have NAFLD or NASH.
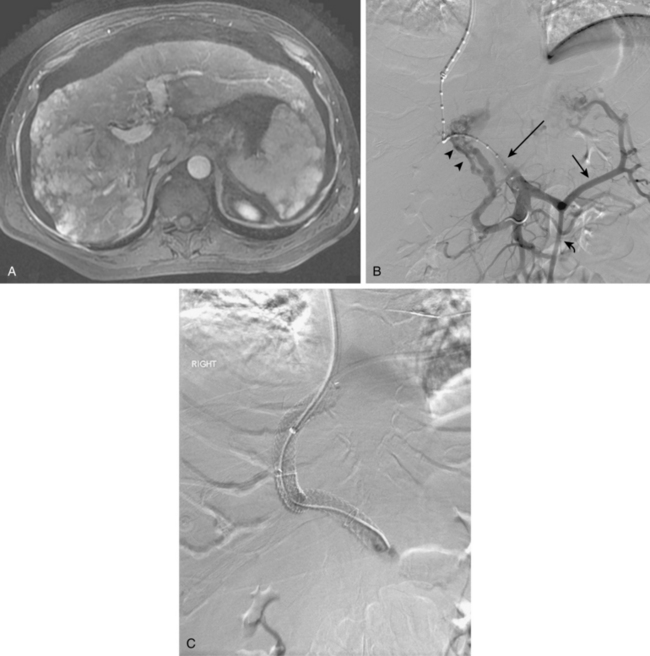
Hyperdynamic portal hypertension, defined as increased flow through the portal venous system in the absence of resistive changes, is an unusual reason for elevated portal pressure. Two lesions that produce this physiology are an arterioportal fistula (which is often the result of penetrating trauma) or rupture of a hepatic artery aneurysm.39 In such cases, embolization of the fistula may be curative.
Clinical features
The most devastating consequence of portal hypertension is bleeding from ruptured gastroesophageal varices that serve as portosystemic collaterals to decompress the fibrotic liver.31 Varices develop in about half of patients with cirrhosis, but only about one third of those will bleed. Hemorrhagic risk correlates with variceal size, intraluminal pressure, and the patient’s Child-Pugh score (Table 12-3). Between 40% and 70% of patients die of the first episode of variceal hemorrhage. Bleeding is unlikely when the portosystemic pressure gradient is less than 12 mm Hg.40
Table 12-3 Modified Child-Pugh Classification for Hepatic Failure
| Determinant | Threshold* |
| Ascites | Controlled medically |
| Encephalopathy | Controlled medically |
| INR | 1.7-2.2 |
| Bilirubin | 2-3 mg/dL |
| Albumin | 2.8-3.5 mg/dL |
| Classification | Points |
| Class A | 5-6 |
| Class B | 7-9 |
| Class C | 10-15 |
* Score 2 points if within threshold, score 1 point if better than threshold, and score 3 points if worse than threshold. INR. International Normalized Ratio.
Ascites is another important complication of portal hypertension. The causes of ascites are manifold. Increased splanchnic blood flow elevates microcirculatory pressures and increases production of lymph, which leaks from the liver and intestines.41 In addition, peripheral arterial dilation (a response to vasoactive factors liberated from the gastrointestinal tract) leads to a reduction in effective plasma volume and retention of salt and water by the kidneys. The hepatic lymphatic system becomes overwhelmed, causing peritoneal leakage and a vicious cycle of further reduction in the plasma volume and worsening ascites.
Cirrhotic patients also are at risk for hepatic encephalopathy, spontaneous bacterial peritonitis, splenomegaly and pancytopenia, hepatocellular carcinoma, and ultimately complete hepatic failure. Less frequent complications include hepatorenal syndrome, hepatopulmonary syndrome, portopulmonary hypertension, and hepatic hydrothorax. Some of these conditions are related to the misregulation of vasodilating and vasoconstricting factors.42Hepatorenal syndrome is characterized by diffuse splanchnic and peripheral vasodilation and decreased effective plasma volume, prompting reflex renal vasoconstriction.43 Whereas the chronic form is treatable, the acute type is almost universally fatal (see later discussion).
Imaging and tissue diagnosis
Hepatic vein manometry
Direct measurement of PV pressure is hardly ever needed for diagnosing portal hypertension; clinical studies have shown that the hepatic vein wedged (HVW) pressure is equal to PV pressure in most patients.44 The difference between HVW and IVC (or right atrial) pressure is the corrected sinusoidal pressure, which reflects the portosystemic gradient. However, the measurements are valid only when the PVs and hepatic sinusoids are in continuity. In patients with extrahepatic PV obstruction, splenic vein obstruction (“segmental” portal hypertension), or presinusoidal portal hypertension, this disconnection leads to a spuriously low HVW pressure. Normally, the portosystemic gradient is less than 5 mm Hg. Portal hypertension is defined as a gradient more than 6 mm Hg. The risk of bleeding from gastroesophageal varices becomes significant when the gradient is greater than 12 mm Hg.40
Hepatic venography
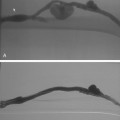
Free hepatic venography is done to assess the hepatic veins if obstruction is suspected, during the transjugular intrahepatic portosystemic shunt (TIPS) procedure, and sometimes during transvenous liver biopsy. Balloon-occluded or catheter-wedged hepatic venography with CO2 is routinely performed before creating TIPS to provide a target for the PV puncture. Hepatic veins have a pinnate (feather-like) branching pattern; portal veins branch dichotomously (Fig. 12-10).
Arteriography and indirect portography
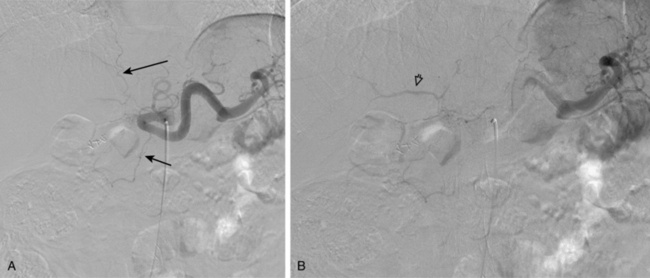
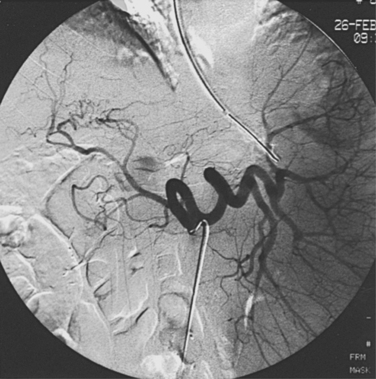
With advanced cirrhosis, the hepatic arteries take on a “corkscrew” appearance because of increased arterial flow and liver shrinkage, and the spleen and splenic artery are enlarged (Fig. 12-11). The demand for hepatic artery flow can become so great that flow in the gastroduodenal artery is reversed. Occasionally, arterioportal shunting is seen (Fig. 12-12).
Figure 12-12 Advanced cirrhosis with global shunting from hepatic artery (A) to portal vein (B) seen on celiac arteriography.
In the early stages of cirrhosis, PV flow is relatively normal. As the disease advances and portal hypertension becomes significant, several changes occur. The most important is the appearance of portosystemic collateral pathways, which include the following principal channels (Fig. 12-13):
Less common routes of decompression are gastric veins to pulmonary or intercostal veins, duodenal varices ultimately draining into the right gonadal vein (Fig. 12-14), left colic vein to left renal vein through the left gonadal vein, ileocolic vein to the IVC through hemorrhoidal veins, and intrahepatic portal venous branches to diaphragmatic veins. Gastroesophageal varices may be present but not seen on indirect portography.
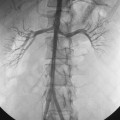
The direction of flow in the PV switches as portal hypertension worsens. With mild cirrhosis, PV flow is hepatopetal (see Fig. 12-3). As resistance increases, bidirectional flow in the PV may develop and the PV may not fill at all. With severe portal hypertension, the PV becomes an outflow conduit for the liver, and flow is hepatofugal (Fig. 12-15).
Transjugular liver biopsy
Patient selection
Tissue samples for histologic diagnosis of diffuse liver disease are usually obtained by percutaneous biopsy. For patients with certain conditions, transhepatic biopsy may be relatively unsafe and transvenous biopsy preferred (Box 12-6). This approach is used also in patients already undergoing hepatic vein catheterization for other reasons (e.g., diagnosis of portal hypertension, during the TIPS procedure).
Technique
From the right (or left) IJ vein, a 40-cm 10-Fr vascular sheath is advanced into the right or middle hepatic vein to the mid-portion of the vessel (Fig. 12-16). A stainless steel stiffening cannula is placed through the sheath. A flexible biopsy needle (e.g., Quick-Core or TLAB Patel set) is then inserted through the cannula and torqued within the hepatic vein until resistance is met. The device is buried in the parenchyma and triggered, and a piece of tissue is removed. Three or four specimens are needed to ensure that sufficient material is obtained for diagnosis.45,46
Results and complications
Liver tissue can be extracted in more than 97% of attempts. It may be slightly more difficult to obtain tissue in liver transplant patients with a “piggyback” type hepatic vein anastomosis47 (see later discussion). Biopsy samples are adequate for pathologic diagnosis 96% to 97% of the time.48–52 The major risk of the procedure is liver capsule perforation. Minor complications (including access site bleeding and cardiac dysrhythmias) are reported in up to 12% of cases. Less than 1% of patients experience serious life-threatening hemorrhage (usually from liver capsule perforation with intraperitoneal bleeding).46,48,49,52
Treatment
Pharmacologic therapy
Prophylactic treatment with beta-adrenergic blocking agents (often combined with isosorbide nitrate) is routinely prescribed in patients with documented gastroesophageal varices to lower portal venous pressure and thereby prevent initial or recurrent bleeding.53,54 If bleeding occurs, emergent management starts with resuscitation, prophylactic antibiotics, and pharmacologic therapy: somatostatin (or its analogue octreotide), vasopressin, or synthetic terlipressin (which constrict mesenteric arteries and reduces portal venous pressure and flow).55–57
Endoscopic treatment
Variceal band ligation or sclerotherapy is highly effective for the initial management and primary or secondary prevention of variceal hemorrhage.53,54,56–59 A sclerosing agent such as ethanolamine oleate or polidocanol injected into or around varices causes variceal thrombosis. Variceal banding is probably safer and more durable.54,57 About 70% to 90% of patients stop bleeding after one or two treatment sessions. However, sclerotherapy does not remedy the underlying hemodynamics of portal hypertension. Rebleeding is observed in about 10% to 15% of cases.31 Serious complications occur about 10% of the time.
Large-volume paracentesis
The standard treatment for cirrhosis-related ascites is sodium and fluid restriction and diuretic therapy. Some cirrhotics with massive (tense) ascites are largely resistant to these measures. Intractable ascites can have a marked impact on overall quality of life. In such cases, frequent large volume paracentesis (>4 to 5 L) is necessary to diminish pain, nausea, and respiratory compromise. The application of volume expanders (e.g., intravenous [IV] albumin infusion) in this setting is controversial. However, large volume paracentesis is inconvenient for patients and does have risk.
Transcatheter variceal embolization
Embolization of the coronary vein was quite popular before the widespread use of endoscopic sclerotherapy.60 Coils, with or without a sclerosing agent, are placed to obstruct the inflow vein. Although immediate results are excellent, rebleeding occurs in more than 50% of cases as new collateral channels develop.61 This procedure has limited use as an adjunct to TIPS placement (see later discussion) and for obliteration of ectopic varices.62
Balloon-occluded retrograde transvenous obliteration of gastric and duodenal varices
In Japan and other parts of Asia, balloon-occluded retrograde transvenous obliteration (BRTO) has become a popular modality for prevention or control of bleeding from isolated variceal clusters.63–65 Gastric and duodenal varices are notoriously difficult to treat with endoscopy because of their location and size. In some individuals, these massive shunts can also cause intractable hepatic encephalopathy.
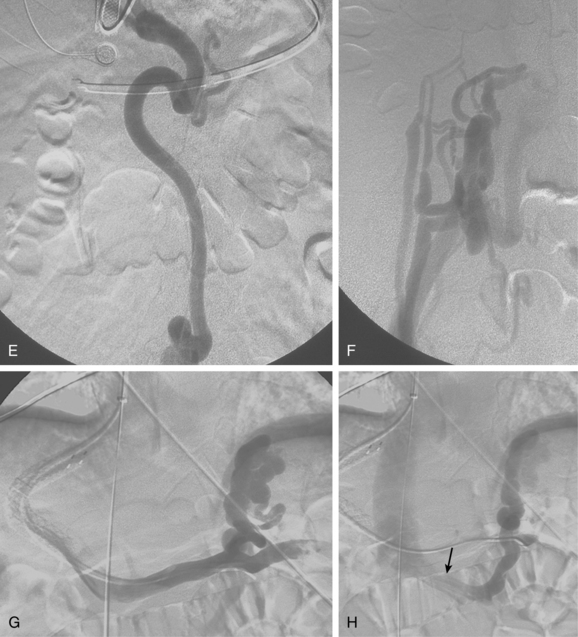
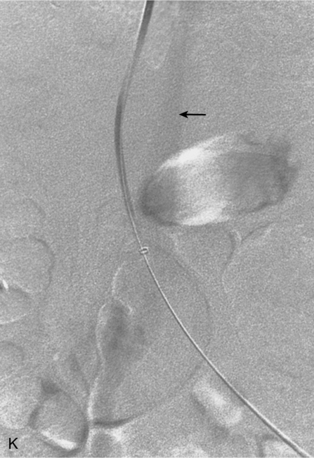
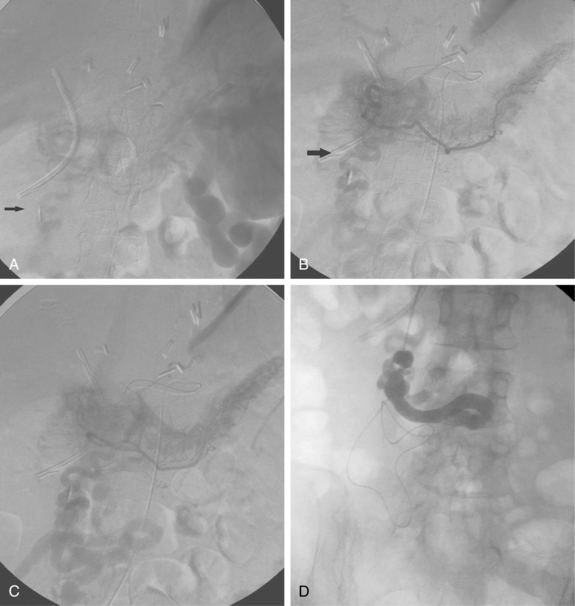
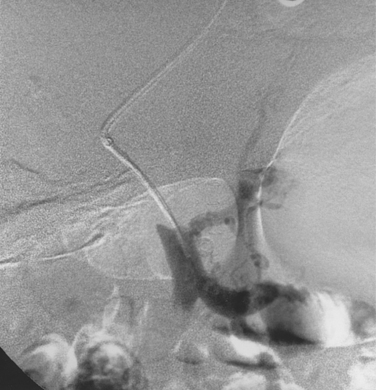
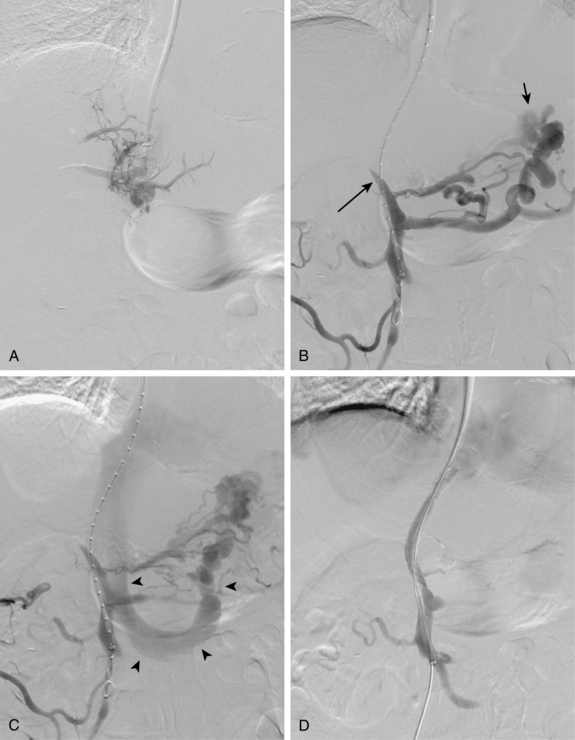
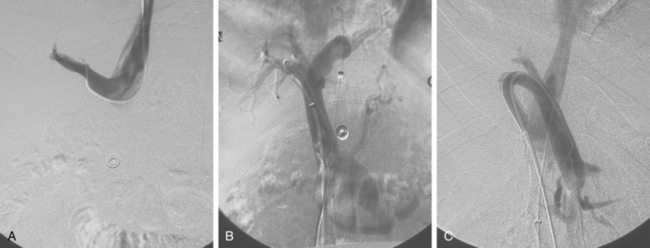
Before BRTO, indirect portography via celiac and SMA arteriography is done to establish the portal venous anatomy and hemodynamics. The outflow vein for the varices (typically the left inferior phrenic/adrenal to left renal vein for gastric varices, right gonadal vein for duodenal varices) is selectively engaged with a diagnostic catheter (see Fig. 12-14). A 6-Fr balloon occlusion catheter (e.g., 11- or 20-mm diameter) is advanced into the main trunk and inflated, and venography is performed to classify the varices and collateral veins.
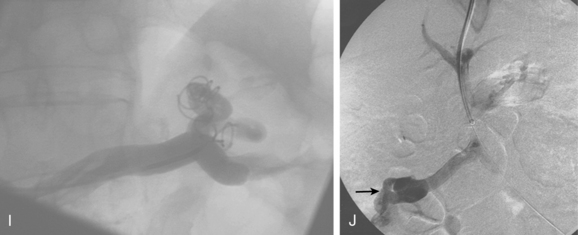
To induce thrombosis of simple varices, 10 mL of ethanolamine oleate 10% mixed with 10 mL of contrast material is slowly injected with the balloon inflated until the dilated veins are completely filled. The agent is left in place from 1 to 24 hours and then aspirated; the balloon is removed. More complex types of variceal communications may require use of a microcatheter or initial partial splenic embolization to reduce flow through the shunts.63,64 Obliteration of varices is documented with contrast CT.
In experienced hands, BRTO is successful in preventing further bleeding and improving encephalopathy in more than 80% of attempts.66 A major drawback to this approach is that purposefully closing down these natural shunts causes elevation of portal pressure and greater risk for esophageal variceal bleeding.67,68 There are some risks to using ethanolamine oleate, including renal failure, pulmonary edema, and anaphylaxis. In Asia it is routine to administer IV haptoglobin, which binds free hemoglobin, to prevent hemolysis-induced kidney damage.
Surgical therapy
In the past, surgical portocaval shunts were commonly recommended for management of these difficult patients.69,70 Side-to-side portacaval and mesocaval shunts are nonselective conduits that direct all portal blood flow away from the liver and return PV pressure to normal levels. The distal splenorenal (Warren) shunt is a selective communication that diverts flow from gastroesophageal varices but maintains intestinal portal flow to the liver. In this procedure, the splenic vein is divided, and the left gastric, right gastric, and gastroepiploic veins are ligated. Surgical mortality rates for all operative shunts are similar (10% to 20%). Rebleeding from varices occurs in about 5% to 15% of cases and is more frequent with selective shunts. Encephalopathy can be problematic.71 In most centers, operative shunts are rarely performed anymore.
Liver transplantation
Liver transplantation is the definitive treatment for relieving portal hypertension from chronic liver disease and provides the best long-term outcome compared with all other methods. The current overall 5-year survival rate for primary liver transplantation in the United States is 79%.72 Not all patients are candidates for transplantation, and donor organs are in short supply.
Transjugular intrahepatic portosystemic shunts (online cases 33 and 78)
Patient selection
Current indications for TIPS have been formulated by several expert groups, including the American Association for the Study of Liver Diseases (AASLD)73–75 (Box 12-7). TIPS is most commonly performed for prevention of recurrent gastroesophageal variceal hemorrhage and management of refractory ascites. Given the inherent risks of the intervention, the fact that many patients with varices will never bleed and that only about half of those who do will rebleed, TIPS is reserved for patients who suffer one or more bleeding episodes that have failed endoscopic methods. However, this stipulation is being questioned. A recent randomized trial in high-risk cirrhotic patients with first variceal hemorrhage compared best medical therapy and endoscopic treatment against initial endoscopic treatment followed by urgent TIPS.76 Rebleeding was significantly less likely and survival significantly longer in the TIPS arm.
Box 12-7 Patient Selection for the Transjugular Intrahepatic Portosystemic Shunt Procedure
Accepted indications
Refractory ascites implies that sodium restriction and maximum diuretic therapy are inadequate therapy. TIPS may afford better quality of life than repeated large-volume paracentesis, but encephalopathy often gets worse and survival is not clearly improved.77,78
Several of the secondary indications for TIPS are worthy of comment.
When counseling patients and referring physicians, it is important to provide a frank assessment of the likelihood of survival following shunt creation. Patients can be stratified in several ways. The severity of liver failure is graded by the Child-Pugh classification (see Table 12-3). The MELD score (Mayo Endstage Liver Disease) has been adopted by many centers to predict outcomes in patients being considered for liver transplantation or TIPS. This score incorporates the serum bilirubin, creatinine, international normalized ratio, and recent need for dialysis (for calculation, go to www.mayoclinic.org/gi-rst/mayomodel6.html). Early mortality after TIPS insertion is especially high in patients with a MELD score greater than 18, Child-Pugh score greater than 12 (class C), APACHE severity of illness score greater than 18 to 20, or bilirubin greater than 3 mg/dL.93–96
Technique
Acute bleeding is controlled with variceal banding or sclerotherapy, systemic terlipressin or octreotide infusion, placement of an esophageal tamponade balloon, or some combination of these measures. Coagulation defects should be corrected and broad-spectrum antibiotics given before the procedure. Some cross-sectional imaging must be obtained beforehand to assess portal vein patency and exclude a large tumor. Color Doppler sonography is adequate. However, three-phase contrast-enhanced CT angiography is certainly better for assessing these parameters (PV patency, status of the hepatic veins, degree of ascites, liver size, presence of liver tumors or polycystic liver disease). Just as important, the operator can determine beforehand the suitability of the individual hepatic veins along with the best trajectory and distance to the portal vein.
The standard access site is the right IJ vein (Fig. 12-19). The left IJ vein is preferred by some operators and should be considered if a second try is made after a failed first attempt. A 40-cm 10-Fr vascular sheath is advanced into the right atrium. Right atrial and IVC pressures are measured. If right atrial pressure is markedly elevated (>20 to 25 mm Hg), the interventionalist should proceed with caution. Fulminant right heart failure can occur after the shunt is created.
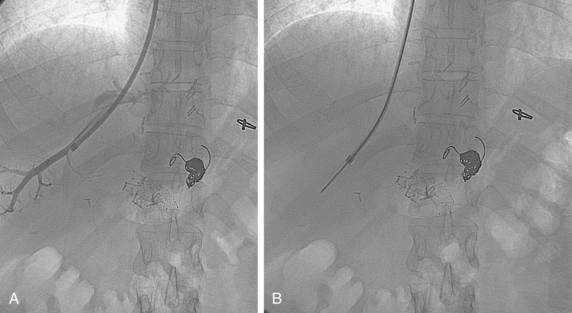
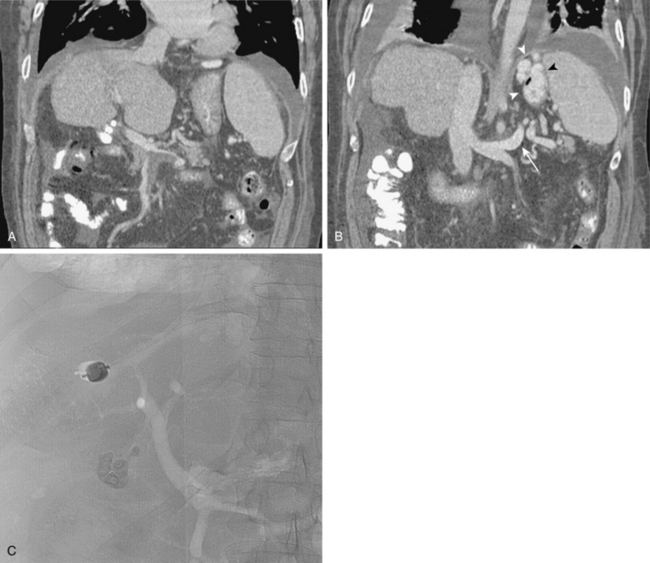
The right or middle hepatic vein is entered with a multipurpose angiographic catheter and steerable guidewire. If the right hepatic vein is small, has a very acute angle with the IVC, or is difficult to catheterize, TIPS can be created from the middle (or even left) hepatic vein. It is important to establish which vein is being used so that the intrahepatic puncture toward the PV is made in the appropriate direction. This step can be accomplished with steep oblique/lateral fluoroscopy or ultrasound interrogation. About 3% of the population has a dominant inferior right hepatic vein. In this situation, TIPS must be formed from the right common femoral vein (Fig. 12-20; see also Fig. 12-8).
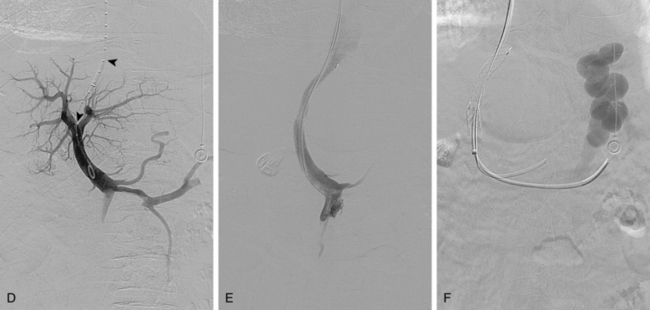
Several methods are used to guide the puncture from the hepatic vein toward the PV (Fig. 12-21). The most popular techniques are as follows:
The site of PV puncture is critical. Because the portal vein bifurcation is extrahepatic in about one half of individuals, entry should be at or peripheral to this point to avoid extrahepatic puncture and the possibility of exsanguinating hemorrhage.16,100
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree