Abstract
Recently, several minimally invasive approaches for interventional therapy of structural heart diseases have been developed. For example, transcatheter aortic valve implantation (TAVI) is an alternative therapeutic option for high-risk patients that serves as an alternative for surgical aortic valve replacement. Also, high-risk patients with mitral valve regurgitation may benefit from interventional mitral valve clipping. Furthermore, left atrial appendage (LAA) closure devices may reduce the risk for thromboembolic complications in patients with atrial fibrillation and a contraindication for oral anticoagulation. Lastly, selected patients may benefit from interventional closure of atrial septal defect (ASD) as well as persistent foramen ovale (PFO). For planning purposes prior to these interventions as well as for peri-interventional guidance, imaging techniques play an important role. Beyond the use of several imaging techniques for structural heart diseases, it has been shown that coronary CT angiography (CCTA) can improve success rates of percutaneous coronary revascularization of chronic total occlusions (CTOs).
Keywords
Imaging, Transesophageal echocardiography, Computed tomography, Aortic stenosis, Mitral valve insufficiency, ASD, PFO, TAVI, MitraClip, PFO closure, LAA closure.
22.1
Introduction
Recently, several minimally invasive approaches for interventional therapy of structural heart diseases have been developed. For example, transcatheter aortic valve implantation (TAVI) is an alternative therapeutic option for high-risk patients that serves as an alternative for surgical aortic valve replacement. Also, high-risk patients with mitral valve regurgitation may benefit from interventional mitral valve clipping. Furthermore, left atrial appendage (LAA) closure devices may reduce the risk for thromboembolic complications in patients with atrial fibrillation and a contraindication for oral anticoagulation. Lastly, selected patients may benefit from interventional closure of atrial septal defect (ASD) as well as persistent foramen ovale (PFO). For planning purposes prior to these interventions as well as for peri-interventional guidance, imaging techniques play an important role. Beyond the use of several imaging techniques for structural heart diseases, it has been shown that coronary CT angiography (CCTA) can improve success rates of percutaneous coronary revascularization of chronic total occlusions (CTOs).
22.2
Transcatheter aortic valve implantation
Over the past years, TAVI has been established as a minimally invasive treatment strategy in patients with severe aortic valve stenosis who are not considered suitable for conventional surgical valve replacement. While new devices are currently being introduced into the market, the most common valves currently used for TAVI are the Sapien® valve (Edwards Lifesciences) and the CoreValve® system (Medtronic, Inc.) ( Figure 22.1 ). These devices are available only in certain sizes and thus are not suitable for all patients. The presence of device-aortic annular mismatch negatively impacts outcome due to the occurrence of aortic regurgitation after implantation, as well as peri-interventional annular rupture.
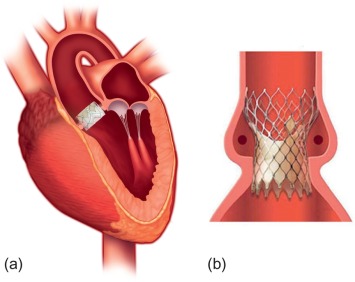
Furthermore, it is crucial to evaluate the most appropriate vascular access pathway (e.g., femoral, apical, or subclavian artery) prior to TAVI. Therefore, imaging techniques are essential for the planning of TAVI. Imaging may also be helpful during valve implantation and for follow-up. The specific imaging protocols are highly dependent on the experience and equipment of the individual institutions. Several centers are using echocardiography for the evaluation of the aortic annulus and root, and initial studies have been based on sizing by ultrasound. However, multidetector computed tomography (MDCT) has become the standard for the evaluation prior to TAVI, especially in many high-volume centers, because the aortic annular shape, which is elliptical rather than circular, can be depicted best using this imaging modality.
Furthermore, tomographic imaging—especially MDCT—has been established for evaluation of the thoracoabdominal aorta and potential access vessels.
Table 22.1 summarizes and compares the importance of several screening CT and echo measurements as well as the principal techniques of preparation and implantation of the Sapien® valve and the CoreValve®.
| Medtronic CoreValve® | Edwards Sapien® | |
|---|---|---|
| Screening CT/Echo Measurement | ||
| LVOT diameter | + | – |
| Mean diameter of the aortic annulus (longitudinal/transverse) | ++ | ++ |
| Circumference of the aortic annulus | + | – |
| Area of the aortic annulus | – | + |
| Distance annulus – coronary ostia | (+) | + |
| Annulus/leaflet calcification | – | + |
| Sinus of valsalva width | – | + |
| Sinus of valsalva hight (annulus – sinutubular junction) | – | + |
| Diameter of the proximal ascending aorta | + | – |
| Peripheral access | ++ | ++ |
| Technique of preparation and implantation | ||
| Balloon valvulopasty | + | + |
| Rapid pacing | – | + |
| Balloon-assisted expansion | – | + |
| Self-Expanding | + | – |
22.2.1
Echocardiography
22.2.1.1
Screening transthoracic and transesophageal echocardiography
Transthoracic echocardiography is the gold standard for the evaluation of the severity of aortic stenosis. Besides aortic valve gradients, the aortic valve orifice area can reliably be determined using the continuity equation (see also Chapter 16 ).
An important challenge for successful trancatheter aortic valve implantation is the correct measurement of specific aortic valve dimensions. Initially these measurements, critical for selection of valve type and size, were performed using 2D transthoracic or transesophageal echocardiography. But because standard transesophageal echocardiography enables the acquisition of high-resolution images, this approach is considered superior to transthoracic echocardiography.
First, the determination of a set of qualitative and semi-quantitative parameters is of importance. These include the assessment of the severity of aortic stenosis as well as concomitant aortic regurgitation. Furthermore, the number, mobility, and structure of the aortic valve cusps, including the extent and location of calcifications are of importance. Currently, the presence of a bicuspid aortic valve is generally considered an exclusion criterion for TAVI because an increased risk for suboptimal valve deployment is assumed. Furthermore, bulky calcified aortic leaflets in close proximity to the coronary ostia might be associated with an increased risk of peri-procedural coronary occlusion, particularly with the use of the Sapien® valve.
Second, the determination of several quantitative parameters affects the selection of valve type and size ( Figure 22.2 , Table 22.1 ):
- (1)
Left ventricular outflow tract (LVOT) diameter.
The LVOT should be evaluated particularly if the CoreValve® is used since significant basal septal hypertrophy might lead to displacement of the prosthesis at the time or shortly after the implantation procedure.
- (2)
Diameter of the aortic annulus.
The most critical measurement for all available valve types is the determination of the diameter of the aortic annulus. The annular diameter predominantly determines the size and type of the prosthesis. An undersized prosthesis might eventually lead to mostly paravalvular regurgitation as well as displacement of the device. An oversized prosthesis might lead to peri-interventional annular rupture. The diameter of the aortic annulus is typically measured at the beginning of systole in the LVOT view (around 110–135°) as the distance from the insertion of the left/non-coronary to the insertion of the right aortic valve cusp. This measurement might be cumbersome in severely calcified valves due to difficulty in identifying the hinge region as well as due to posterior acoustic attenuation.
- (3)
Distance from the aortic annulus to the coronary ostia.
The distance from the aortic annulus to the coronary ostia should be obtained to compare this measurement with the lengths of the aortic valve cusps. These measurements should be acquired in the LVOT view, particularly if the Sapien® valve is used. The length of the aortic valve cusps is usually shorter than the annular-ostial distance. However, these measurements are important since the (calcified) aortic valve cusps are crushed against the aortic wall during the implantation procedure. This might compromise the coronary ostia potentially resulting in life threatening complications.
- (4)
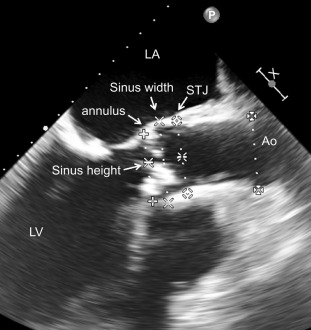
Sinus of Valsalva width and height.
The width and height of the sinus of Valsalva can be measured in the LVOT view. It is assumed that enough space is required to accommodate the native valve cusps to not compromise the coronary ostia. The width of the sinus of Valsalva is measured as the diameter of the aortic root at the midsinusal level. The height of sinus of Valsalva represents the distance between the aortic valve annulus to the level of the sinotubular junction. These measurements are of theoretical concern in particular for the Sapien® valve and should be compared to the dimension of the device intended to implant.
- (5)
Diameter of the proximal ascending aorta.
The diameter of the ascending aorta should be measured at its proximal part about 1 cm distal to the sinotubular junction. This measurement is of importance if the CoreValve® device is taken into consideration since it contains a broader upper segment that secures the device in the ascending aorta. Thus, in patients with ascending aortic diameters > 45 mm, the CoreValve® should not be implanted.
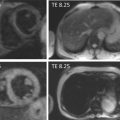
2D transesophageal echocardiography does not accurately depict the actual oval shape of the aortic valve annulus. This is why novel three-dimensional imaging modalities including 3D TEE and MDCT were introduced. Studies comparing 2D TEE to 3D TEE as well as MDCT show significantly larger annular diameters using these novel technologies, suggesting 2D TEE underestimates annular dimensions [ , ].
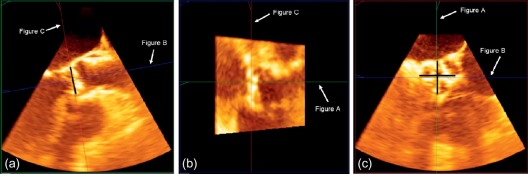
Aortic annular assessment by 3D transesophageal echocardiography requires the acquisition of a 3D volume. Subsequently, offline analysis with multiplanar reconstruction of this dataset enables the accurate assessment of the shape of the aortic annulus, including its maximum and minimum diameter ( Figure 22.3 ). Husser et al. reported significantly smaller annulus diameters and areas using 3D TEE compared to MDCT. However, 3D TEE sagittal diameters correlated well with MDCT [ ].
Although there is no defined gold standard for the acquisition of aortic valve measurements, an increasing number of centers favor performing MDCT scans over echocardiographic measurements. There are a number of advantages using this approach that will be described in the following section.
22.2.1.2
Peri-interventional transesophageal echocardiography
One major advantage of transesophageal echocardiography is its availability during the interventional procedure. In case of doubt, all the abovementioned measurements, including the measurements acquired by 3D TEE, can readily be repeated. Furthermore, several steps of the implantation procedure can be supported by transesophageal echocardiography. First, TEE can assist in balloon and prosthesis sizing and positioning during the implantation procedure. Second, prosthesis function, including residual aortic regurgitation, can be evaluated immediately after implantation. Regarding aortic regurgitation, paravalvular regurgitation can be distinguished from valvular regurgitation, and allows a decision regarding further measures, such as balloon-postdilatation, in particular, in the case of significant paravalvular regurgitation.
However, peri-interventional TEE often necessitates the use of general anesthesia during the implantation procedure. This is why a number of interventionalists prefer performing the TAVI without TEE guidance, irrespective of the valve type intended for use.
22.2.1.3
Follow-up transthoracic echocardiography
Transthoracic echocardiography is used for follow-up evaluation of valve function, including ejection fraction. Usually mean gradients < 10 mmHg can be measured in case of proper valve function. Pressure half time is the preferred parameter for the assessment of mostly paravalvular regurgitation. In case of good valve function, yearly follow-up visits should be considered.
22.2.2
Computed tomography
MDCT enables a comprehensive 3D assessment of the aortic valve, aortic root, and the thoracoabdominal aorta as well as its iliofemoral branches for planning purposes prior to TAVI procedures [ ]. Therefore, in many centers, MDCT has become a standard modality for this indication. Detailed evaluation of the aortic valve annulus is crucial for accurate sizing of the valve prosthesis. Several studies have demonstrated that the aortic valve annulus is usually oval in shape. The CT examination can be performed in different ways, and there are multiple dimensions that can be measured and reported. So far, there is no single validated CT-methodology for the evaluation of patients referred for TAVI.
22.2.2.1
Image acquisition
A CT examination for TAVI planning needs to cover the aortic root, the entire thoracoabdominal aorta, and the iliac arteries as well as the proximal femoral arteries. If the subclavian arteries are considered for access, then the scan needs to be further extended cranially. Detailed measurements have to be performed, therefore, so the reconstructed slices thickness should be 1.0 mm or less. To allow for a high image quality of the aortic root without major motion artifacts, ECG-synchronized image acquisition is required in this region, either using retrospective ECG gating or prospective ECG triggering. Because a number of studies have shown a larger size of the aortic annulus during systole compared to diastole, systolic image acquisition might be preferable to lower the risk of paravalvular regurgitation. Image data of the remaining parts of the thoracoabdominal aorta and iliofemoral branches do not need to be acquired in an ECG-synchronized fashion, thereby limiting radiation exposure, scan duration, and required amount of contrast agent. The dedicated scan protocol highly depends on the CT system. When using a single-source MDCT system with 64 detectors or fewer, it is reasonable to split the examination into two parts. After an ECG-synchronized scan of the heart and the aortic root, a helical scan of the remaining volume is performed without ECG triggering, which requires a second or long infusion of contrast agent. When using a wide-detector CT system, it might be feasible to image the entire volume with ECG synchronization. Second- and third-generation dual-source CT systems can perform a prospectively ECG-triggered high-pitch spiral scan, which allows a TAVI planning CT examination at a very low radiation and contrast medium exposure. Whereas radiation exposure is not of major concern in TAVI patients, mainly considering their advanced age in general, keeping the amount of contrast agent low is important in patients with severe aortic valve stenosis. First of all, patients with severe aortic valve stenosis have a high risk of cardiac decompensation when subjected to a large amount of injected volume. Second, many patients with severe aortic valve stenosis suffer from renal insufficiency, predisposing them for contrast-induced nephropathy. Typically an amount of 80–120 ml contrast agent is administered for TAVI planning CT. However, it has been shown that the amount of contrast agent might be reduced to 60 ml or even below using dedicated scan protocols [ ].
22.2.2.2
Image evaluation
Most importantly, the CT assessment prior to TAVI involves the measurements of the aortic valve annulus for correct prosthesis sizing, its distance to the coronary artery ostia, and evaluation of the thoracoabdominal aorta and its iliofemoral branches.
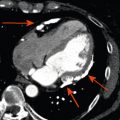
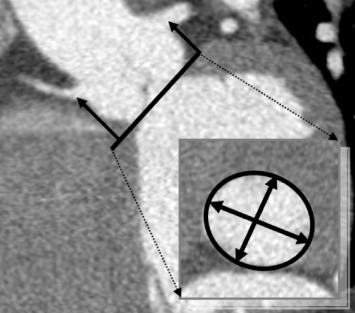
Exact determination of the aortic valve annulus dimensions is of major importance because the annulus might be too large or too small for available prosthesis sizes. Furthermore, prosthesis undersizing or oversizing might result in complications such as paravalvular regurgitation or rupture of the aortic root. For evaluation of the aortic valve annulus, a double-oblique plane is required that includes all three lowest insertion points of the aortic valve leaflets ( Figure 22.4 ). Using this double-oblique image data, there are three different approaches of deriving the mean diameter of the oval-shaped aortic valve annulus [ ] (see Figure 22.4 ). First, it may be calculated as the average of the long and short diameter. Second, it may be derived from the aortic valve annulus circumference, which is divided by π . Third, it may be calculated from the planimetrically measured annulus area as: <SPAN role=presentation tabIndex=0 id=MathJax-Element-1-Frame class=MathJax style="POSITION: relative" data-mathml='meandiameter=2×area÷π’>meandiameter=2×(area÷π)‾‾‾‾‾‾‾‾‾√meandiameter=2×area÷π
mean diameter = 2 × area ÷ π
. Investigating the reproducibility of aortic annulus assessments, it has been shown that the determination of the area-derived diameter was associated with the best reproducibility, which may indicate that this CT-based method should be preferred over others [ ]. Although there is no broad consensus about the ideal timing of CT data acquisition during the heart cycle, and although the quantification of the mean annulus diameter did not differ between diastole and systole, systolic measurements are usually preferred over diastolic measurements [ ].
When the transcatheter aortic valve prosthesis is implanted, the native aortic valve leaflets are displaced against the wall of the sinus of Valsalva with the risk of coronary artery occlusion. To minimize the risk of coronary artery occlusion, usually a minimum distance of 11–14 mm between the aortic valve annulus and the coronary artery ostia is generally recommended, depending on prosthesis type and size. Therefore, these distances need to be reported from ECG-synchronized 3D image data of the aortic root (see Figure 22.4 ). Length or calcification of the aortic valve cusps may also be predictive for coronary artery occlusion.
Commonly, a transfemoral access route is being chosen for the TAVI procedure. Alternatively, a trans-subclavian, transaortic, or transapical (Sapien®) approach may be possible. To determine the optimal access pathway with the lowest risk for vascular complications, a detailed evaluation of the thoracoabdominal aorta and its potential access branches is necessary in every patient. Computed tomography can reliably detect risk factors for vascular complications such as insufficient vessel diameter compared to delivery system, the degree of atherosclerosis, and vessel tortuosity, or kinking. For a transfemoral TAVI approach a minimum vessel diameter between 6 and 8 mm—depending on prosthesis type and size—is required to minimize the risk of vascular complications.
Furthermore, it has been shown that CT image data can be helpful to derive the optimal aortic annulus plane for fluoroscopy. When performing TAVI, a fluoroscopic projection is required in which the valve prosthesis is orthogonal to the native valve plane, preferably with all valve cusps separated. Traditionally, this projection has been derived from repeat aortograms. However, CT offers three-dimensional data of the orientation of the aortic root and therefore is able to predict the optimal fluoroscopic projection for the TAVI procedure. Using CT data has been shown to increase the rate of achieving an optimal fluoroscopic projection angle for implantation [ ] and to reduce the number of performed aortic angiograms and amount of contrast agent needed [ ].
In addition, CT has been demonstrated to be of prognostic value in TAVI patients. First, the use of CT for aortic annulus sizing was associated with a reduced rate of more than mild paravalvular regurgitation after valve implantation, compared to 2D echocardiography [ ]. Second, the extent of aortic valve calcification predicts postprocedural aortic regurgitation, complications during implantation, and 1-year mortality as well as improvement in NYHA-class after TAVI procedure [ ].
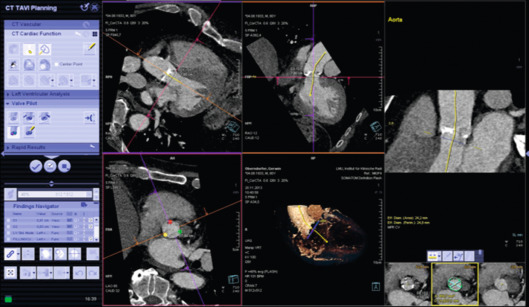
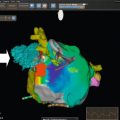
Today, a variety of dedicated CT software packages are available that facilitate an automated accurate analysis of aortic valve dimensions as well as of vascular tree for preprocedural planning ( Figure 22.5 ). Some software packages also include peri-procedural hybrid CT/fluoroscopic imaging for selection of an optimal fluoroscopic angulation angle and for guiding valve deployment.