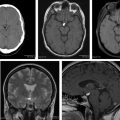
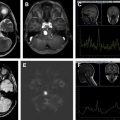
Primary central nervous system lymphomas are aggressive, high-cell-density tumors. There is recent increase in their incidence in immunocompetent patients. Knowledge of imaging findings on computed tomography and conventional MR imaging is important to suggest the diagnosis. Moreover, information obtained from advanced MR imaging techniques, such as diffusion-weighted imaging, diffusion tensor imaging, MR spectroscopy, perfusion-weighted imaging, and dynamic contrast-enhanced studies, increases diagnostic confidence and helps distinguish them from other aggressive intracranial tumors. This article discusses typical imaging findings of primary and secondary central nervous system lymphomas on computed tomography and conventional MR imaging, advanced MR imaging techniques, and changes related to steroid therapy.
Restricted diffusion may not be demonstrated in lymphoma after steroid therapy.
Restricted diffusion may not be demonstrated in lymphoma after steroid therapy.
The peak age for CNS lymphoma in the non-AIDS population is during the sixth decade of life with men affected more than women.
Restricted ADC may resolve after steroids.
Summary
When PCNSL is suspected, contrast-enhanced MR imaging is the technique of choice. Secondary CNS lymphomas present as meningeal metastases in two-thirds of patients and as parenchymal metastases in one-third. In PCNSL, almost all patients have parenchymal lesions. Parenchymal lymphomas have a predilection for the periventricular and superficial regions, often abutting the ventricular or meningeal surfaces.
Although CNS lymphomas may have characteristic imaging findings on traditional MR imaging, no technique unequivocally differentiates CNS lymphoma from other brain lesions. Advanced MR imaging techniques, such as DWI, DTI, MRS, PWI, and DCE studies, help establish a diagnosis of CNS lymphoma and distinguish lymphomas from other aggressive primary brain tumors, such as GBM. If ADC suggests high cellular density and no elevation of rCBV is seen, consider lymphoma as the main diagnosis.
Following administration of steroids, lymphomas may partially or completely regress, restricted ADC may resolve, and a previously hyperperfused lesion may demonstrate low rCBV.
Funding Sources: None.
Conflict of Interest: None.
References
- 1. Atlas S.W.: Extra-axial brain tumors. In Atlas S.W. (eds): Magnetic resonance imaging of the brain and spine, 2nd edition. Philadelphia: Lippincott Raven, 1996. pp. 446-448
- 2. Jellinger K., Radszkiewicz T., and Slowk F.: Primary malignant lymphoma of the central nervous system in man. Acta Neuropathol 1975; undefined: pp. 95
- 3. Baumgartner J., Rachin J., Beckstead J., et al: Primary central nervous system lymphomas: natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg 1990; 73: pp. 206-211
- 4. Atlas S.W.: Intra-axial brain tumors. In Atlas S.W. (eds): Magnetic resonance imaging of the brain and spine, 2nd edition. Philadelphia: Lippincott Raven, 1996. pp. 404-407
- 5. Hochberg F.H., and Miller D.C.: Primary central nervous system lymphoma. J Neurosurg 1988; 68: pp. 835-853
- 6. Nasir S., and Deangelis L.M.: Update on the management of primary CNS lymphoma. Oncology 2000; 14: pp. 228-237
- 7. Forsyth P.A., and DeAngelis L.M.: Biology and management of AIDS-associated primary CNS lymphomas. Hematol Oncol Clin North Am 1996; 10: pp. 1125-1134
- 8. Diamond C., Taylor T.H., Aboumrad T., et al: Changes in acquired immuno- deficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer 2006; 106: pp. 128-135
- 9. Besson C., Goubar A., Gabarre J., et al: Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood 2001; 98: pp. 2339-2344
- 10. Greenberg J.O., and Polachini I.: Intracranial neoplasms. In Greenberg J.O. (eds): Neuroimaging. A companion to Adams and Victor’s principles of neurology. Mc-Graw-Hill, Inc, 1995. pp. 340-342
- 11. Russel D.S., and Rubinstein L.J.: Pathology of tumors of the central nervous system. Baltimore (MD): Williams & Wilkins, 1989.
- 12. Clark W., Callihan T., Schwartzberg L., et al: Primary intracranial Hodgkin’s lymphoma without dural attachment. J Neurosurg 1992; 76: pp. 692-695
- 13. Jack C.R., O’Neill B.P., Banks P.M., et al: Central nervous system lymphoma: histologic types and CT appearance. Radiology 1988; 167: pp. 211-215
- 14. Okazaki H., and Scheithauer B.W.: Atlas of neuropathology. New York: Gower, 1988.
- 15. Enzmann D.R., Tokye K.C., and Hayward R.: CT in leptomeningeal spread of tumor. J Comput Assist Tomogr 1978; 2: pp. 448-455
- 16. Haldorsen I.S., Espeland A., and Larsson A.M.: Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 2011; 32: pp. 984-992
- 17. Nacif M.S., Jauregui G.F., Mello R.A.F., et al: Linfoma adrenal primário bilateral com envolvimento do sistema nervoso central: relato de caso. Radiol Bras 2005; 38: pp. 235-238
- 18. Erdag N., Bhorade R.M., and Alberico R.A.: Primary lymphoma of the central nervous system. Typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol 2001; 176: pp. 1319-1326
- 19. Tang Y.Z., Booth T.C., Bhogal P., et al: Imaging of primary central nervous system lymphoma. Clin Radiol 2011; 66: pp. 768-777
- 20. Haque S., Law M., Abrey L.E., et al: Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am 2008; 46: pp. 339-361
- 21. Zambrano AD, Berkowitz F. Primary central nervous system lymphoma: what the radiologist needs to know. Presented at the ASNR Annual Meeting. Quebec (Canada), May 17–22, 2014. p. eEdE18.
- 22. Lee H.Y., Kim H.S., Park J.W., et al: Atypical imaging features of Epstein-Barr virus–positive primary central nervous system lymphomas in patients without AIDS. AJNR Am J Neuroradiol 2013; 34: pp. 1562-1567
- 23. Oyama T., Ichimura K., Suzuki R., et al: Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol 2003; 27: pp. 16-26
- 24. Nakamura S., Jaffe E.S., and Swerdlow S.H.: EBV positive diffuse large B-cell lymphoma of the elderly. In Swerdlow S.H., Campo E., and Harris N.L. (eds): WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon (France): IARS Press, 2008. pp. 243-244
- 25. Eichler A.F., and Batchelor T.T.: Primary central nervous system lymphoma: presentation, diagnosis and staging. Neurosurg Focus 2006; 21: pp. E15
- 26. Go J.L., Lee S.C., and Kim P.E.: Imaging of primary central nervous system lymphoma. Neurosurg Focus 2006; 21: pp. E4
- 27. Choi C.Y., Lee C.H., and Joo M.: Lymphomatosis cerebri. J Korean Neurosurg Soc 2013; 54: pp. 420-422
- 28. Keswani A., Bigio E., and Grimm S.: Lymphomatosis cerebri presenting with orthostatic hypotension, anorexia, and paraparesis. J Neurooncol 2012; 109: pp. 581-586
- 29. Poon T., Matoso I., Tchertkoff V., et al: CT features of primary cerebral lymphoma in AIDS and non-AIDS patients. J Comput Assist Tomogr 1989; 13: pp. 6-9
- 30. Stadnick T.W., Demaerel P., Luypaert R.R., et al: Imaging tutorial: differential diagnosis of bright lesions on diffusion-weighted MR images. Radiographics 2003; 23: pp. e7
- 31. Wang S., Kim S., Chawla S., et al: Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2011; 32: pp. 507-514
- 32. Calli C., Kitis O., Yunten N., et al: Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol 2006; 58: pp. 394-403
- 33. Cha S.: Neuroimaging in neuro-oncology. Neurotherapeutics 2009; 6: pp. 465-477
- 34. Brandão L.A., Shiroishi M.S., and Law M.: Brain tumors: a multimodality approach with diffusion-weighted imaging, diffusion tensor imaging, magnetic resonance spectroscopy, dynamic susceptibility contrast and dynamic contrast-enhanced magnetic resonance imaging. In modern imaging evaluation of the brain, body and spine. Magn Reson Imaging Clin N Am 2013; 21: pp. 203-207
- 35. Guo A.C., Cummings T.J., Dash R.C., et al: Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 2002; 224: pp. 177-183
- 36. Yamasaki F., Kurisu K., Satoh K., et al: Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005; 235: pp. 985-991
- 37. Toh C.H., Castillo M., Wong A.C., et al: Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol 2008; 29: pp. 471-475
- 38. Stadnik T.W., Chaskis C., Michotte A., et al: Diffusion-weighted MR images of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol 2001; 22: pp. 969-976
- 39. Sugahara T., Korogi Y., Kochi M., et al: Usefulness of diffusion-weighted MRI with echo-planar techniques in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999; 9: pp. 53-60
- 40. Kono K., Inoue Y., Nakayama K., et al: The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001; 22: pp. 1081-1088
- 41. Horger M., Fenchel M., Nãgele T., et al: Water diffusivity: comparison of primary CNS lymphoma and astrocytic tumor infiltrating the corpus callosum. AJR Am J Roentgenol 2009; 193: pp. 1384-1387
- 42. Batra A., and Tripathi R.P.: Atypical diffusion-weighted magnetic resonance findings in glioblastoma multiforme. Australas Radiol 2004; 48: pp. 388-391
- 43. Hakyemez B., Erdogan C., Yildirim N., et al: Glioblastoma multiforme with atypical diffusion-weighted MR findings. Br J Radiol 2005; 78: pp. 989-992
- 44. Toh C.H., Chen Y.L., Hsieh T.C., et al: Glioblastoma multiforme with diffusion-weighted magnetic resonance imaging characteristics mimicking primary brain lymphoma. Case report. J Neurosurg 2006; 105: pp. 132-135
- 45. Baehring J.M., Bi W.L., Bannykh S., et al: Diffusion MRI in the early diagnosis of malignant glioma. J Neurooncol 2007; 82: pp. 221-225
- 46. Chang Y.W., Yoon H.K., Shih H.J., et al: MR imaging of glioblastoma in children: usefulness of diffusion/perfusion-weighted MRI and MR spectroscopy. Pediatr Radiol 2003; 33: pp. 836-842
- 47. Ahn S.J., Shin H.J., Chang J.H., et al: Differentiation between primary cerebral lymphoma and glioblastoma using the apparent diffusion coefficient: comparison of three different ROI methods. PLoS One 2014; 9: pp. e112948
- 48. Barajas R.F., Rubenstein J.L., Chang J.S., et al: Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 2010; 31: pp. 60-66
- 49. Valles F.E., Perez-Valles C.L., Regalado S., et al: Combined diffusion and perfusion MR imaging as biomarkers of prognosis in immunocompetent patients with primary central nervous system lymphoma. AJNR Am J Neuroradiol 2013; 34: pp. 35-40
- 50. Punwani S., Taylor S.A., Saad Z.Z., et al: Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging 2013; 40: pp. 373-385
- 51. Beppu T., Inoue T., Shibata Y., et al: Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J Neurooncol 2003; 63: pp. 109-116
- 52. Kinoshita M., Hashimoto N., Goto T., et al: Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 2008; 43: pp. 29-35
- 53. Vargova L., Homola A., Zamecnik J., et al: Diffusion parameters of the extracellular space in human gliomas. Glia 2003; 42: pp. 77-88
- 54. Zamecnik J.: The extracellular space and matrix of gliomas. Acta Neuropathol 2005; 110: pp. 435-442
- 55. Brandão L., and Domingues R.: Intracranial neoplasms. In McAllister L., Lazar T., and Cook R.E. (eds): MR spectroscopy of the brain. Philadelphia: Lippincott Williams & Wilkins, 2004. pp. 130-167
- 56. Brandão L.A., and Castillo M.: Adult brain tumors: clinical applications of magnetic resonance spectroscopy. Neuroimag Clin N Am 2013; 23: pp. 527-555
- 57. Knopp EA. Advanced MR imaging of tumors using spectroscopy and perfusion. Presented at the 39th Annual Meeting of the American Society of Neuroradiology. Boston, April 23–27, 2001.
- 58. Castillo M.: Proton MR spectroscopy of common brain tumors. Neuroimaging Clin N Am 1998; 8: pp. 733-752
- 59. Toh C.H., Wei K.C., Chang C.N., et al: Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR Am J Neuroradiol 2013; 34: pp. 1145-1149
- 60. Liao W., Liu Y., Wang X., et al: Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol 2009; 50: pp. 217-225
- 61. Cha S., Knop E.A., Jhonson G., et al: Intracranial mass lesions: dynamic contrast enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 2002; 223: pp. 11-29
- 62. Rowland L.A., Pedley T.A., and Merritt H.H.: Distinguishing of primary cerebral lymphoma from high grade glioma with perfusion-weighted magnetic resonance imaging. Neurosci Lett 2003; 338: pp. 119-122
- 63. Weber M.A., Zoubaa S., Schileter M., et al: Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 2006; 66: pp. 1899-1906
- 64. Patankar T.F., Haroon H.A., Mills S.J., et al: Is volume transfer coefficient (Ktrans) related to histologic grade in human gliomas? AJNR Am J Neuroradiol 2005; 26: pp. 2455-2465
- 65. Cha S., Yang L., Johnson G., et al: Comparison of microvascular permeability measurements, K trans, determined with conventional steady-state T1-weighted and first pass T2*-weighted MR imaging methods in gliomas and meningiomas. AJNR Am J Neuroradiol 2006; 27: pp. 409-417
- 66. Eby N.L., Grufferman S., Flanelly C.M., et al: Increasing incidence of primary brain lymphoma in the US. Cancer 1998; 62: pp. 2461-2465
- 67. DeAngelis L.M.: Current management of primary central nervous system lymphoma. Oncology (Williston Park) 1995; 9: pp. 63-71
- 68. Samani A., Davagnanam I., Cockerell O.C., et al: Lymphomatosis cerebri: a treatable cause of rapidly progressive dementia. J Neurol Neurosurg Psychiatry 2015; 86: pp. 238-240
- 69. ØStergaard L., Hochberg F.H., Rabinov J., et al: Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg 1999; 90: pp. 300-305
- 70. DeAngelis L.M., Yahalom J., Heinemann M.-H., et al: Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology 1990; 40: pp. 80-86
- 71. Henry J.M., Heffner R.R., and Dillard S.H.: Primary malignant lymphomas of the nervous system. Cancer 1974; 34: pp. 1293-1302
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree