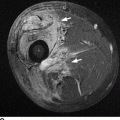
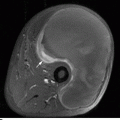
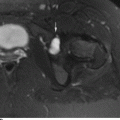
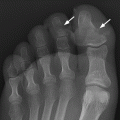
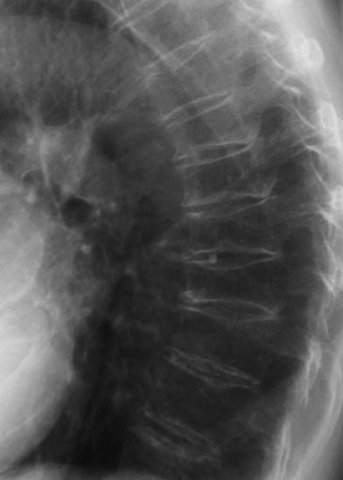
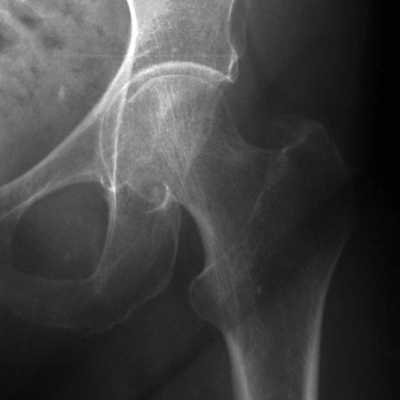
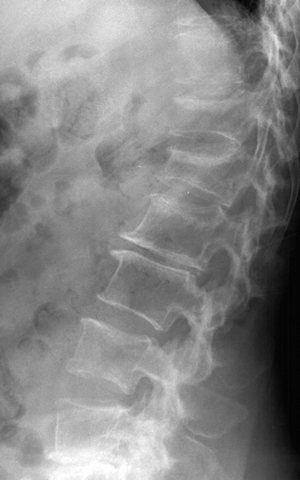
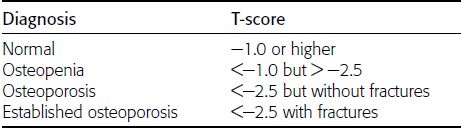
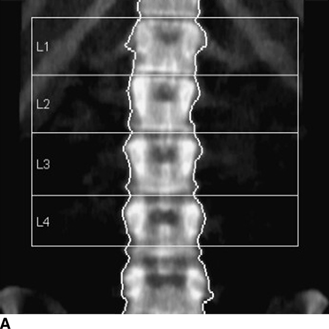
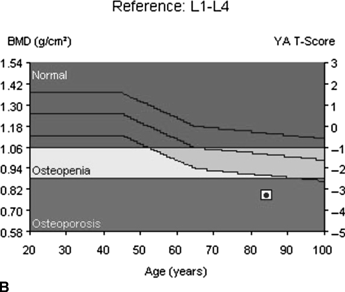
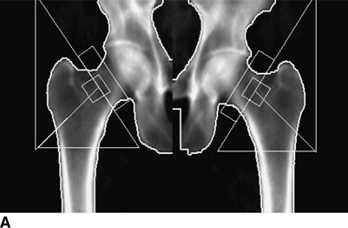
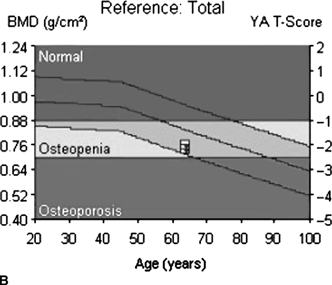
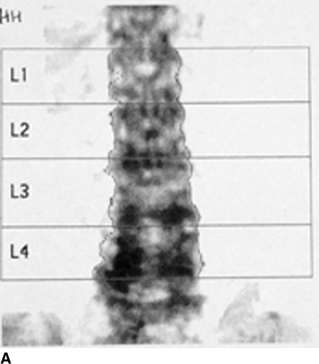
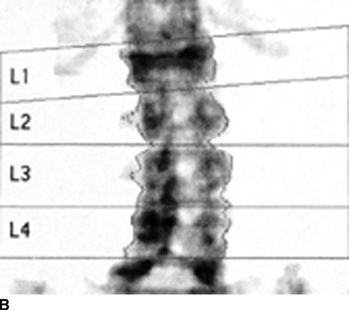
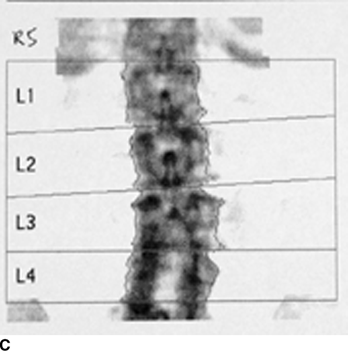
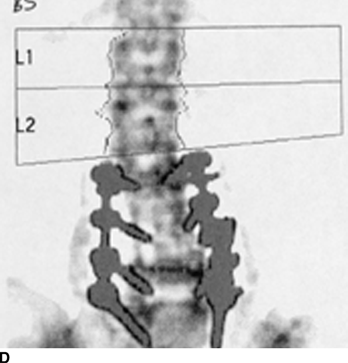
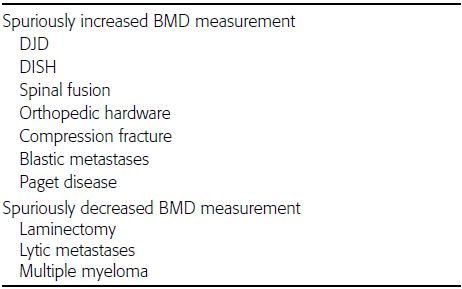
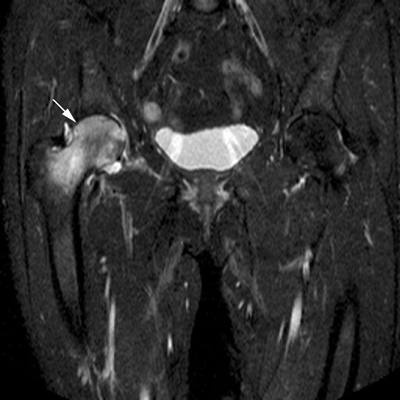
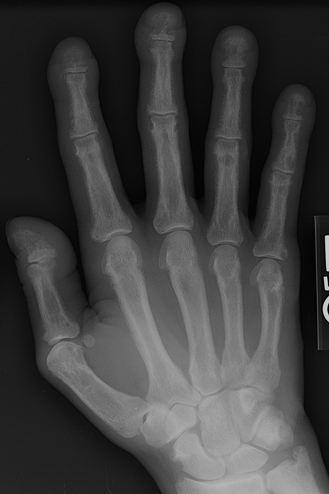
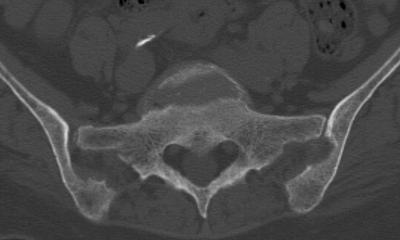
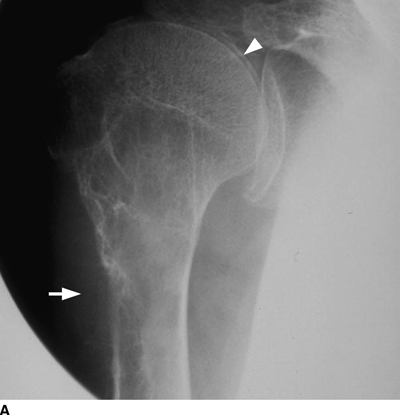
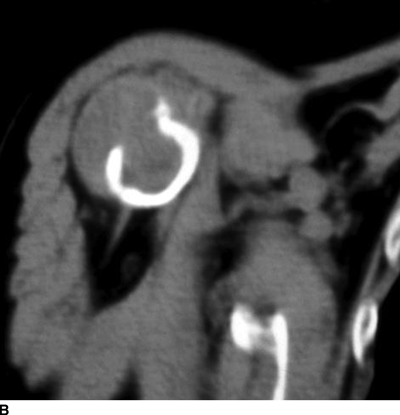
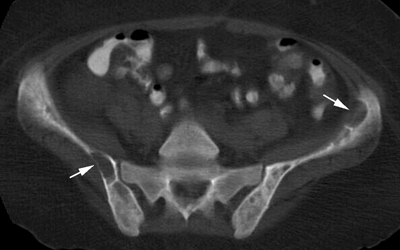
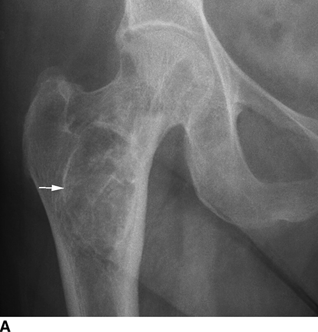
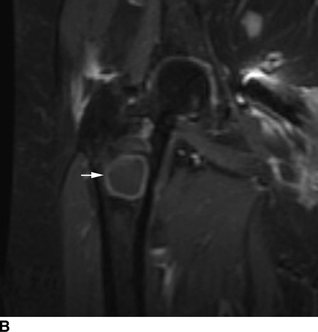
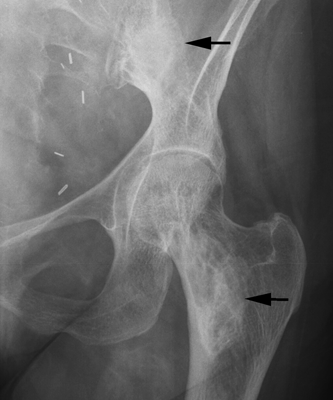
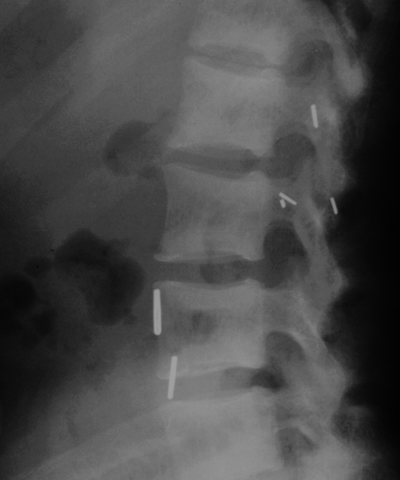
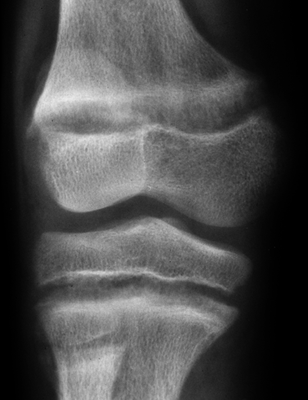
CHAPTER 15 This chapter describes the radiology of many systemic, metabolic, and endocrine conditions that have bone or soft-tissue manifestations. Osteoporosis is the most common metabolic bone disease. Osteoporosis is characterized by generalized loss of mass from an otherwise normal bone. This loss of bone substance results in microarchitectural deterioration of the trabecular structure, increased bone fragility, and increased risk of fracture. Osteoporosis may be considered to be primary or secondary. Primary osteoporosis, also called involutional osteoporosis, is idiopathic in etiology. Secondary osteoporosis has a known, underlying etiology. More than 95% of adults with osteoporosis have involutional osteoporosis (also called idiopathic or primary osteoporosis). There are two main clinical types: (a) postmenopausal and (b) senile or age related. Postmenpausal osteoporosis is characterized by accelerated bone loss, mainly trabecular, and is caused by factors related to menopause. Age-related osteoporosis is characterized by slowly progressive bone loss, mainly cortical, and is caused by factors related to aging. The pathogenesis of osteoporosis is incompletely understood; it seems to involve not only excessive bone resorption but also impaired bone formation. Deteriorating bone strength results in increased vulnerability to traumatic and fatigue fractures. Most fractures of the spine, proximal femur, and distal radius in adults older than 50 years of age are associated with osteoporosis (see Chapter 1). In postmenopausal osteoporosis, vertebral crush fractures and distal radius fractures are the most common; in age-related osteoporosis, multiple vertebral wedge fractures and hip fractures are the most common. Fractures and the resulting loss of function are the major cause of morbidity and mortality in osteoporosis. The prevalence of involutional osteoporosis in the United States is estimated at 28 million, or approximately 10% of the entire population. Risk factors for developing involutional osteoporosis include advancing age; female gender; white or Asian race; family history; thin, small bones; menopause; low dietary calcium; low dietary vitamin D; excessive alcohol, caffeine, and salt; smoking; and sedentary lifestyle. As the proportion and absolute numbers of the population in the United States older than 50 years of age increase, the prevalence of involutional osteoporosis may be expected to increase substantially. The radiographic hallmark of osteoporosis is osteopenia, the increased radiolucency of bone. Osteopenia may not be recognizable on plain films until 30% to 50% of the bone mineral has been lost. A coarsened trabecular pattern results from the loss of smaller trabeculae, making the remaining ones more prominent. Linear, bandlike, or spotty radiolucent areas may also be seen. Cortical thinning can be a generalized, uniform, slowly progressive process (Fig. 15.1). Cortical bone loss may also be manifested by scalloped, endosteal erosions, intracortical radiolucent areas or striations (intracortical tunneling), or subperiosteal erosions. Vertebral deformities are related to insufficiency fractures of the end plates. These can have the form of biconcave depressions of contiguous superior and inferior end plates (so-called fish or codfish vertebrae, named for their resemblance to vertebrae in codfish), anterior wedge fractures, crush fractures of entire vertebral bodies, and increased thoracic kyphosis (dowager hump) (Figs. 15.2 and 15.3). The net result is progressive loss of stature. FIGURE 15.1. Osteoporosis of the hip with thin cortex and loss of trabecular bone in the neck. FIGURE 15.2. Osteoporosis in the spine with central endplate depressions of the vertebral bodies at multiple levels. FIGURE 15.3. Osteoporosis with multiple anterior wedge fractures in the thoracic spine. Bone mineral densitometry (BMD) is the best noninvasive method for the diagnosis of osteoporosis in asymptomatic patients. BMD can also be used to estimate the risk of fracture and monitor patients receiving therapy for osteoporosis. The availability of effective therapies for osteoporosis—including estrogen, bisphosphonates, and other pharmacologic agents—has increased the usefulness of screening patients with various risk factors. The absolute amount of bone mineral is predictive of fracture risk. Radiographic measurement of BMD accurately and precisely assesses bone mineral content. Dual-photon absorptiometry (DPA) and dual-energy X-ray absorptiometry (DXA) use a dual-energy radionuclide and X-ray source, respectively, to measure the bone density of the lumbar spine or hip. DPA and DXA measure the absorption of photons by the bone and provide a combined measurement of cortical and trabecular bone density. Quantitative CT measures the trabecular bone density in lumbar vertebral bodies by comparing the CT numbers of trabecular bone with the CT numbers of standardized solutions of calcium suspensions. Quantitative ultrasound measures the sound conduction in bone as a correlate of BMD. Broadband ultrasound attenuation has also been correlated with BMD as another method of quantitative ultrasound. Measures of BMD can be compared to populations matched for age, sex, and race to establish criteria for normality and abnormality. The relative risk of an osteoporotic fracture can also be estimated for various levels of BMD by comparing the incidence of fractures in large populations with BMD measurements. Because cortical and trabecular bone may not be involved to the same extent, methods that measure one or the other, or both, may not yield identical results. DXA works on the physical principle that bone mineral (predominantly calcium) and soft tissue (predominantly fat and water) attenuate X-rays of high and low energy to different degrees. By measuring the differential absorption of X-rays of high and low energy, the absorption due to bone mineral alone can be determined, and from that, the absolute amount of bone mineral in the path of the X-rays can be calculated. Measuring the BMD at the spine and proximal femur (central measurement) is considered more useful clinically than measuring the BMD in the extremities (peripheral measurement). DXA provides measurements that reflect the BMD of trabecular and cortical bones together. Clinical interpretation of the results of DXA is based on population statistics. An individual’s BMD measurement from DXA is compared with those of normal populations. The consensus criteria used for clinical diagnosis are those of the World Health Organization (WHO) (Table 15.1). According to WHO criteria, an individual’s BMD is compared with those of a population of normal, young adults to derive a “T-score.” The T-score is related to the number of standard deviations from the young, normal population mean the individual’s measurement falls, so that a T-score of -2.0 would be 2 standard deviations below the mean of the young, normal population. Particular levels of bone mineral have been established as corresponding to the disease. A T-score of less than -1.0 but greater than -2.5 corresponds to a diagnosis of osteopenia. A T-score of less than -2.5 corresponds to a diagnosis of osteoporosis. If fractures are present in a patient with a T-score of less than -2.5, the diagnosis is established osteoporosis. The comparison of an individual’s BMD to an age-matched population (the Z-score) is not used in WHO criteria. There is significant controversy regarding the particular normal, young adult populations on whom the T-scores are based with regard to sample size, gender, and race. The use of the terms osteopenia and osteoporosis may also lead to confusion unless the context is clear; when these terms are used in conjunction with DXA, they should only refer to diagnoses according to WHO criteria. TABLE 15.1 WHO Diagnostic Criteria for Involutional Osteoporosis The T-score is the number of standard deviations from the mean relative to a normal young adult population. In current practice, the DXA diagnosis of osteopenia and osteoporosis is based on BMD measurements of the lumbar spine and the proximal femur. In the spine, the frontal projection is used, and, ideally, an average of L1 through L4 is used to obtain the T-score (Fig. 15.4). If it is not possible to use L1 through L4, any two or three consecutive L1 through L4 vertebrae can be used. Use of a single vertebra is considered unreliable. In the hip, a region of the femoral neck is used (Fig. 15.5). The DXA report should include, as a minimum, a description of the basis of the measurement, the T-score, the relative fracture risk associated with the T-score, and the diagnosis according to WHO criteria. FIGURE 15.4. DXA of the lumbar spine showing osteoporosis. A: Image shows regions of interest. B: Graphic shows bone mineral density relative to young-adult and age-matched reference populations. The T-score was -3.3, diagnostic of osteoporosis. FIGURE 15.5. DXA of the hips showing osteopenia. A: Image shows regions of interest. B: Graphic shows bone mineral density relative to young-adult and age-matched reference populations. The T-score on the right was -2.0, and the T-score on the left was -2.2, both diagnostic of osteopenia. Because DXA is a projection technique that measures the sum of the bone mineral between the X-ray source and the detector, disease or postsurgical conditions that increase or decrease the attenuation of the X-rays may compromise accuracy (Fig. 15.6). The most common condition leading to spurious BMD measurements (Table 15.2) is degenerative spine disease, in which sclerosis and osteophytes increase the BMD measurement. Similarly, diffuse idiopathic skeletal hyperostosis (DISH) may increase the BMD measurement. Other common conditions that affect DXA measurements include compression fractures, laminectomy, metal artifacts and bone graft from spine fusion, focal sclerotic lesions such as blastic metastases or Paget disease, and focal lytic lesions such as lytic metastases or multiple myeloma. Similar conditions may affect DXA measurements of BMD at the hip. FIGURE 15.6. DXA artifacts. A: Degenerative spine disease with endplate sclerosis and osteophytosis. B: Compression fracture at L1 and degenerative changes at L4. C: Laminectomy and posterolateral fusion at L4. D: Lumbar spine fusion with hardware. TABLE 15.2 Common Potential Causes of Spurious BMD Measurements in the Spine Secondary osteoporosis is the loss of bone mass from an otherwise normal bone as a result of a known cause. Secondary osteoporosis may manifest acutely or chronically, in a regional or systemic distribution. DXA and other methods for determining BMD may be applied to patients with chronic, systemic, secondary osteoporosis. DXA may be used to establish the diagnosis and to follow therapy. Common causes of secondary osteoporosis are listed in Table 15.3. More detailed descriptions of these conditions may be found in the appropriate portions of this book. TABLE 15.3 Some Causes of Secondary Osteoporosis in Adults Acute osteoporosis accompanies fracture healing as a normal physiologic response to hyperemia. It may also accompany immobilization and disuse, both common features of healing fractures, as well as conditions such as reflex sympathetic dystrophy (also called complex regional pain syndrome). On radiographs, acute osteoporosis may be recognized in cancellous bone by subcortical resorption of trabeculae, resulting in subcortical lucency that parallels the cortical surface. On CT, the bone resorption tends to appear patchier (Fig. 15.7). FIGURE 15.7. Acute osteoporosis, associated with a healing femoral shaft fracture, demonstrated on CT scan. Transient regional osteoporosis typically presents with monoarticular joint symptoms. It is characterized by a rapidly developing osteoporosis affecting periarticular bone that is self-limited and reversible and has no definite inciting event. There are two clinical syndromes that occur in adults, both more common in men. The first clinical syndrome is transient osteoporosis of the hip. This presents with the rapid onset of severe hip pain without a precipitating event. It is usually unilateral, involving either the right or the left hip in men or usually the left hip in women. The pain is self-limited but aggravated by activity and regresses in 2 to 6 months without permanent sequelae. Imaging may show a rapidly developing periarticular osteoporosis, particularly in the femoral head, that returns to normal after the resolution of symptoms. MRI shows diffuse edema and hip effusion but no infarction. Bone scans show increased activity consistent with osteoporosis. The condition is thought to have a neurogenic origin, possibly related to reflex sympathetic dystrophy. The second clinical syndrome is regional migratory osteoporosis (Fig. 15.8). This usually involves the lower extremities of adults. Local pain and swelling develop rapidly, last up to 9 months, and then disappear only to be followed by the involvement of other regions months to years later. Within weeks of the onset of each episode, periarticular osteoporosis is evident. This condition may begin as transient osteoporosis of the hip. FIGURE 15.8. Transient osteoporosis of the hip. Coronal T2-weighted MRI with fat suppression shows amorphous bone marrow edema (arrow) in the right proximal femur. The skeleton is a metabolically active reservoir of calcium and phosphorus in the extracellular space. These minerals are maintained in tiny hydroxyapatite crystals that have in aggregate a vast surface area and are rapidly and freely exchanged into the extracellular fluid space. When necessary, the skeleton gives up calcium to maintain the correct serum levels. The ionic concentration of calcium is generally determined by the renal glomerular filtration rate, tubular reabsorption of calcium, and the formation and resorption of bone. The serum calcium level is tightly controlled by parathyroid hormone and 1,25-dihydroxyvitamin D. Calcium can be directly released from the hydroxyapatite crystal or liberated by osteoclastic destruction of bone. Bone formation and destruction are closely coupled in a healthy person. Hyperparathyroidism stimulates osteoclastic resorption of bone. The excess parathyroid hormone can be a result of primary or secondary hyperparathyroidism. Primary hyperparathyroidism results in hypercalcemia and is caused by excess parathyroid hormone production from diffuse parathyroid hyperplasia or autonomously functioning parathyroid adenomas (single or multiple). Secondary hyperparathyroidism is a response to sustained hypocalcemia that is typically caused by chronic renal failure or gastrointestinal malabsorption. Patients with long-standing secondary hyperparathyroidism may develop relatively autonomous parathyroid function or tertiary hyperparathyroidism. Diagnosis of these disorders is made by clinical and laboratory findings, including direct measurement of serum calcium and parathyroid hormone levels. Although radiographic changes may occur at many sites, they are best detected and monitored in the hands. Skeletal surveys of the whole body are generally unnecessary. Radiologic signs of hyperparathyroidism include bone resorption, brown tumors, bone sclerosis, and chondrocalcinosis. Bone resorption occurs at all surfaces, including subperiosteal, intracortical (along haversian systems), endosteal, trabecular, subchondral, and subligamentous locations. Subperiosteal bone resorption is virtually diagnostic of hyperparathyroidism. Seen best and most frequently along the radial aspect of the phalanges of the hands, especially the middle phalanges of the index and middle fingers, subperiosteal bone resorption may also be evident at the phalangeal tufts (Fig. 15.9). Bone resorption at other surfaces is nonspecific. Subchondral bone resorption may lead to articular disease (Fig. 15.10). Brown tumors are focal areas of bone resorption where the bone has been replaced by fibrous tissue and osteoclasts (Fig. 15.11). Brown tumors may have the appearance of aggressive, focally destructive bone lesions such as metastases, but the associated presence of other radiographic changes of hyperparathyroid bone disease should clarify the situation. Brown tumors may be single or multiple in number and central, eccentric, or cortical in location (Figs. 15.12 and 15.13). Brown tumors heal by ossification (Fig. 15.14). Widespread sclerosis of bone in hyperparathyroidism occurs by an uncertain mechanism and may be prominent in the axial skeleton, especially the skull and spine. Widespread sclerosis is common in secondary hyperparathyroidism. When the spine is involved, horizontal bands of sclerosis in the vertebral bodies adjacent to the vertebral end plates may result in a rugger jersey appearance (Fig. 15.15). Chondrocalcinosis, or calcification of the cartilage, is associated with the combination of primary hyperparathyroidism and calcium pyrophosphate dihydrate crystal deposition disease (see Chapter 13). This combination is found in 18% to 40% of primary hyperparathyroidism cases. FIGURE 15.9. Secondary hyperparathyroidism. Subperiosteal bone resorption is evident throughout the hand and more advanced on the radial aspect. FIGURE 15.10. Hyperparathyroidism with sacroiliac joint bone resorption. Axial CT scan shows subchondral bone resorption and widening of the sacroiliac joints. FIGURE 15.11. Brown tumor in hyperparathyroidism. A: AP radiograph shows osteoporosis and destructive lesion (arrow) in proximal humerus. Chondrocalcinosis is present (arrowhead). B: CT shows mass destroying cortex. FIGURE 15.12. Secondary hyperparathyroidism with brown tumors (arrows) and bone resorption on CT. FIGURE 15.13. Brown tumor of the proximal femur. A: AP radiograph shows a lytic lesion (arrow) in the intertrochanteric region of the proximal femur. B: Axial T1-weighted MRI with fat suppression following a gadolinium injection shows rim enhancement (arrow) in this cystic lesion. FIGURE 15.14. Healing brown tumors involving the pelvis and proximal femurs (arrows). FIGURE 15.15. Secondary hyperparathyroidism. Osteosclerosis with greater density at the endplates (rugger jersey spine). Sometimes, the distinction between primary and secondary hyperparathyroidism can be suggested. The following combination of findings is highly suggestive of primary hyperparathyroidism: subperiosteal bone resorption, bone resorption at other sites, bony sclerosis, and chondrocalcinosis. In secondary hyperparathyroidism, there is an increased frequency of vascular and soft-tissue calcification, more commonly and more widespread bone sclerosis, and a decreased frequency of chondrocalcinosis. Secondary hyperparathyroidism is common; primary hyperparathyroidism is uncommon. Rickets and osteomalacia are childhood and adult manifestations, respectively, of a systemic disease in which the calcification of osteoid is deficient. The final common pathway in both conditions is the lack of available calcium or phosphorus (or both) for mineralization of osteoid. In rickets, the predominant effect is on the growth plates; in osteomalacia, the predominant effect is on remodeling of the mature bone. When rickets or osteomalacia occurs in conjunction with chronic renal failure, the condition is called renal osteodystrophy. Dietary deficiency of vitamin D, usually coupled with inadequate exposure to sunlight so that photochemical synthesis of vitamin D in the skin does not occur, results in reduced gastrointestinal calcium absorption, hypocalcemia, and secondary hyperparathyroidism to mobilize calcium from the skeleton. Pure vitamin D deficiency–induced rickets and osteomalacia are relatively rare in the United States except among immigrants, food faddists, the institutionalized elderly people, and patients on total parenteral nutrition. Other causes include failure of enzymatic conversion of 25-hydroxyvitamin D to its physiologically more active metabolite 1,25-dihydroxyvitamin D, end-organ insensitivity to 1,25-dihydroxyvitamin D, genetic and acquired renal tubular reabsorptive defects, and gastrointestinal malabsorption of dietary calcium or phosphorus. In the United States, gastrointestinal malabsorption from a variety of etiologies is the most common cause of rickets and osteomalacia. Rickets and osteomalacia may occur in association with polyostotic fibrous dysplasia and neurofibromatosis and may also be caused by chronic use of anticonvulsant medications or aluminum-containing antacids. In rickets, there is widening of the growth plate because of continued cartilage growth in the absence of normal mineralization and ossification (Fig. 15.16). Radiographic findings are most apparent in the most active regions of growth, and the uncalcified cartilage may become quite bulky. Frequent sites of radiographic abnormalities include the costochondral junctions of ribs, the distal femur, both ends of the tibia, the proximal humerus, the distal radius, and the ulna. Irregular, disorganized mineralization of the zone of provisional calcification creates a frayed appearance. Mechanical stress on the thickened growth plate may lead to widening, cupping, and bowing deformities. Bone texture (trabecular pattern) appears coarsened, and there is delayed appearance of ossification centers. Rachitic bone is less resistant to bending and shearing loads, and stress fractures and bowing deformities are common. Transverse zones of lucency on the concave side of long bones, called Milkman pseudofractures or Looser zones, are focal collections of nonmineralized osteoid; they probably do not represent insufficiency injuries. After the initiation of successful treatment of rickets, the uncalcified osteoid calcifies, so that the zone of provisional calcification appears as a wide band that narrows the growth plate to its normal thickness (Fig. 15.17). Ossification of nonmineralized subperiosteal osteoid is apparent as new periosteal bone. FIGURE 15.16. Renal osteodystrophy with thickened growth plates and irregular ossification at the interface with the metaphysis.
Metabolic and Systemic Conditions
 OSTEOPOROSIS
OSTEOPOROSIS
Primary Osteoporosis (Involutional Osteoporosis)
Bone Mineral Densitometry
Secondary Osteoporosis

Acute, Transient, Regional, and Migratory Osteoporosis
 DISEASES OF MINERAL METABOLISM
DISEASES OF MINERAL METABOLISM
Hyperparathyroidism
Rickets, Osteomalacia, and Renal Osteodystrophy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree