A variety of nontuberculous mycobacteria (NTMB) can cause pulmonary infections, with important differences in epidemiology, microbiology, host response, and treatment options across the various species. The severity of an infection caused by NTMB is largely determined by the immune status of the individual and whether coexisting disease is present. More complex manifestations of NTMB are now recognized, such as the overlapping pathology of hypersensitivity pneumonitis and infection seen in patients with so-called hot tub lung.
Prevalence and Epidemiology
NTMB can be classified as “environmental opportunistic” mycobacteria because they are ubiquitous, not transmitted from person to person, and occur in water (natural sources such as rivers and domestic drinking water), dust, and soil. The Mycobacterium avium complex is the most readily recovered from the natural environment and from patients with NTMB disease. There are also differences in prevalence of individual NTMB species among geographic regions; for example, Mycobacterium malmoense is more common in Europe than in North America.
In some parts of the world, water supplies have been investigated as a potential source of NTMB; even when water appears to be sterile, a “biofilm” (layering on the internal surfaces of water pipes) may be positive for NTMB. A biofilm deposit of NTMB may also be present on dental drilling equipment or bronchoscopes, creating resistance to disinfection. NTMB occur both in natural bodies of water and in artificial sources, including swimming pools and whirlpool baths. Inhalation of droplets containing NTMB may result in a hypersensitivity pneumonitis (see later section Hot Tub Lung ). Environmental exposure to NTMB may occur in water-damaged buildings, in which high concentrations of NTMB may be generated, particularly during building demolition.
The relatively low incidence of clinically significant infection caused by NTMB (on the order of 0.5 to 10 per 100,000 population, depending on the geographic location), despite an undoubtedly high level of environmental exposure, indicates that NTMB are of relatively low pathogenicity. Nevertheless, the reported incidence of NTMB infections continues to rise, in part as a consequence of the increasing awareness and identification of NTMB as human pathogens.
Classification of Nontuberculous Mycobacteria
Decades ago the genus Mycobacterium consisted of approximately 30 species; today, more than 150 species are recognized. Many of these species, some of which have exotic names (e.g., M. conspicuum, M. heckeshornense, and M. mucogenicum ), can cause pulmonary disease. A wide variety of other diseases can be caused by NTMB; for example, M. avium can be responsible for conditions ranging from tenosynovitis to bacteremia in AIDS patients, as well as the more widely recognized pulmonary manifestations.
NTMB can be divided into slowly growing or rapidly growing groups. Of the many slow-growing NTMB, the species most frequently associated with human lung disease are M. avium and M. intracellulare, nearly identical organisms that are usually referred to as the M. avium complex (MAC). M. chelonae, M. fortuitum, and M. abscessus comprise the three important rapidly growing species, all of which can cause pulmonary disease. The NTMB can be further subdivided on the basis of colony characteristics, including pigment production. A prolonged period needs to elapse before the culture characteristics of slow-growing NTMB become evident. By contrast, polymerase chain reaction assays are rapid and highly specific for some NTMB species, although false-positive results can arise in the presence of clinically nonsignificant NTMB.
Clinical Presentation: Colonization Versus Infection
Because NTMB are so widespread in the environment, respiratory secretions (sputum specimens or bronchoalveolar lavage samples) will occasionally contain NTMB, which may represent a contaminant as opposed to a clinically significant infection. Colonization can also occur, with NTMB recovered from an individual on more than one occasion without accompanying features of pulmonary infection. A diagnosis of clinically significant NTMB infection depends on supportive clinical findings. American Thoracic Society (ATS) guidelines specify three criteria for establishing a diagnosis of NTMB disease: (1) the clinical manifestations and time course must be compatible with NTMB-related pulmonary infection, (2) the radiographic findings must be compatible, and (3) the organism should be recovered in sufficient number (either from sputum or as a positive culture from bronchial washings). There are difficulties with these criteria; for example, patients with radiographically progressive MAC infection may be asymptomatic, and conversely, symptoms may be a reflection of the underlying pulmonary disease that predisposes an individual to colonization/infection by NTMB rather than being caused by the NTMB itself. Radiographic findings of MAC infection may be subtle, and high-resolution computed tomography (HRCT) findings may be nonspecific. Integration of all available information from the clinical features, imaging (particularly HRCT), and microbiology is needed to make the sometimes very difficult distinction between colonization and clinically important infection.
Pathology
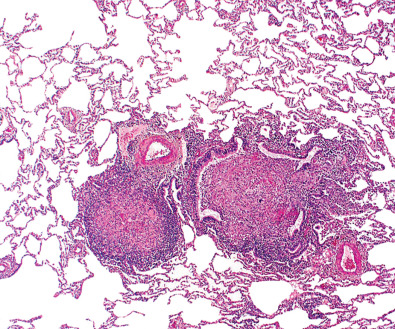
Pathogenic mechanisms differ among the various NTMB species, and an understanding of these differences helps explain the clinical situations in which NTMB cause important infection. The slow-growing NTMB generally breach the host’s mucosal surface, the gastrointestinal or the respiratory tract in the case of MAC. Organisms introduce themselves into alveolar epithelial cells and replicate without causing a major inflammatory response. Once across the “host barrier,” the more virulent mycobacteria are able to replicate within monocytes and macrophages. A granulomatous host response eventually occurs. In one of the histopathologic studies of pulmonary NTMB infection, Fujita and colleagues identified extensive peribronchial granulomas ( Fig. 11.1 ) and associated ulceration of bronchial walls; from these features they concluded that MAC caused, rather than colonized, preexisting bronchiectasis.
The immune status of the host has a profound effect on the potential expression of disease caused by NTMB. The predisposition to disseminated NTMB disease in HIV-infected patients has been recognized for many years. The development of NTMB infection, notably MAC, in apparently otherwise healthy individuals may be due to defects in interferon production or specific interferon receptors. More obvious perturbations of the host’s immune state, such as steroid treatment and coexisting disease, are discussed in a later section.
The rapidly growing NTMB ( M. fortuitum, M. chelonae, and M. abscessus ) most frequently cause infections of soft tissues, particularly wounds. Pulmonary infections are often part of a disseminated picture in which there is multiorgan involvement. In this situation the degree of host immunity is a crucial determinant in the severity of the illness and prognosis. In addition, M. fortuitum and M. chelonae may cause diffuse pulmonary infection in patients with esophageal motility disorders, such as achalasia, hiatal hernia, dysmotility after stroke, or colonic interposition.
Mycobacterium avium-intracellulare Complex
Pulmonary infection by MAC is quite common in clinical practice but frequently overlooked. The radiologist is in a good position to raise the possibility of the diagnosis, especially because the clinical manifestations tend to be nonspecific. The radiographic manifestations of MAC are modulated by the immune status of the host and the presence or absence of preexisting lung disease; thus the spectrum can range from a few inconspicuous nodules to destructive cavitary disease on a background of emphysema. Chest radiography has a valuable role in monitoring patients with a confirmed diagnosis of MAC infection, whereas HRCT may detect earlier disease and suggest the diagnosis.
Manifestations of Mycobacterium avium-intracellulare Complex Infection
Radiography.
Many of the early radiographic studies of NTMB did not attempt to distinguish among the various species, so it is difficult to determine whether there are definite differences in radiographic manifestations among the species. Any putative differences may, in fact, reflect the characteristics of the host rather than the specific NTMB species; in addition, more than one species of NTMB may be present.
The more florid cavitary form of disease (called the “classic” form for its resemblance to cavitary tuberculosis), reported in the earlier literature, probably reflects the use of sputum culture as the primary tool for confirming the diagnosis, which means that only those with a relatively high burden of NTMB in their sputum would have been included in these series. This type of disease is most commonly encountered in elderly men, frequently with a background of pulmonary disease, especially chronic obstructive pulmonary disease (COPD) ( Fig. 11.2 ). At the other end of the radiographic spectrum are the normal or nearly normal radiographic findings in patients with confirmed MAC infection. In one of the largest series, Christensen and coauthors reported that 7 of 114 patients with sputum culture–positive MAC infection had normal chest radiographs, and 7 had a reticulonodular pattern. The majority of individuals in early radiographic series had focal pulmonary abnormalities ranging from small nodules to large cavities. The high frequency of radiographically evident cavities (in the study by Christensen and colleagues, nearly 98% of individuals had identifiable cavities) has probably declined with the trend toward earlier diagnosis. There is a tendency for cavities caused by NTMB to be smaller and thinner walled (average diameter, 2.5 cm) than those caused by M. tuberculosis (average diameter, 6 cm), although the overlap in size is so great that differentiation cannot be made on the basis of these size criteria.
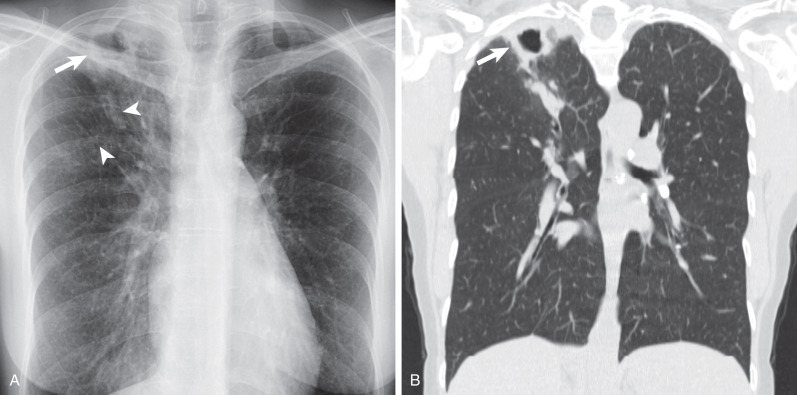
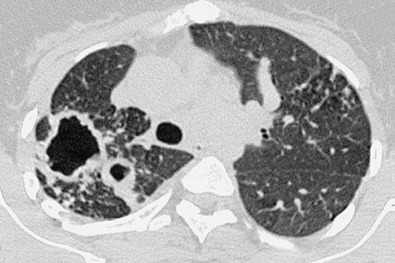
The less florid form of MAC infection, called the “nonclassic” form, is now more frequently recognized than the “classic” form and is seen most often in elderly women with no obvious preexisting lung disease or clearly characterized immune defect. In such individuals the radiographic manifestations of MAC infection include normal radiographs, small inconspicuous nodules (ranging from a few millimeters to approximately 1.5 cm), and patchy foci of consolidation with bronchiectasis, often with a right middle lobe and lingular predominance ( Fig. 11.3 ). Small nodules often represent endobronchial spread (corresponding to the HRCT finding of a tree-in-bud pattern [ Fig. 11.4 ]). Indolent disease usually associated with elderly women may wax and wane without treatment, and some nodules may disappear spontaneously, only to reappear at different locations months or years later. In the series of Woodring and associates, the average time over which there was definite radiographic progression in patients with MAC infection was 6.4 years, and in one particular case, 12 years elapsed after diagnosis before radiographic progression occurred. In the same study the authors remarked on the delay (1–16 years) between initial evaluation and the diagnosis of MAC infection, again highlighting the nonspecific nature of the clinical and radiographic findings. An uncommon manifestation of MAC infection is as a single focal pulmonary mass or solitary nodule on a chest radiograph. Such nodules and masses range in size from 1 to 5 cm, and whether they represent a truly solitary lesion, in the absence of CT corroboration, is uncertain.
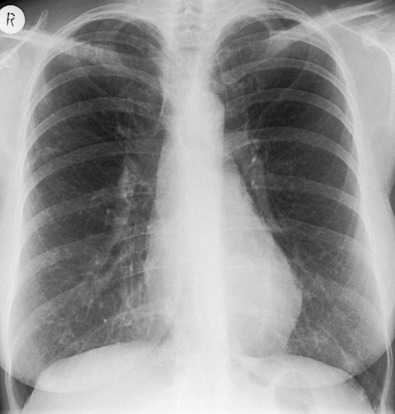
Bronchiectasis in the right middle lobe and lingula ( Fig. 11.5 ) may be particularly prominent in some elderly women with the nonclassic form of disease, and the term Lady Windermere syndrome has been coined for this pattern.
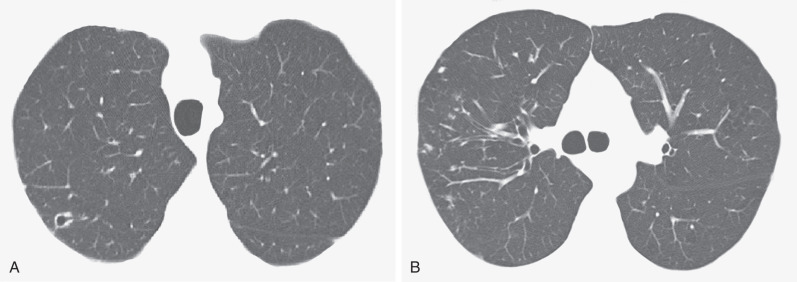
Computed Tomography.
CT is sensitive for detection of pulmonary MAC infection and may show relatively widespread involvement even when radiographs are unimpressive. Although individual CT features of MAC are nonspecific, the combination of CT signs (see the next box), particularly when seen in an elderly woman, is highly suggestive of the diagnosis ( Fig. 11.6 ; also see Figs. 11.4 and 11.5 ). Indeed, the CT appearance may be sufficiently characteristic of MAC infection even in the absence of any overt clinical features.
Dominant Features
- •
Bronchiectasis
- •
Usually multilobar with a right middle lobe and lingular predominance
- •
Usually cylindrical and mild to moderate in severity; rarely cystic or end-stage bronchiectasis
- •
- •
Nodules
- •
Small centrilobular branching nodules (tree-in-bud pattern)
- •
Occasional random nodules up to approximately 2 cm in diameter, occasionally cavitary
- •
Rare Features
- •
Fibrosis associated with bronchiectasis, pleural thickening or pleural effusion, mediastinal lymphadenopathy
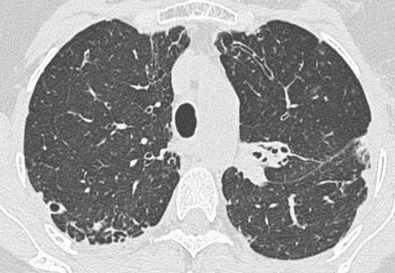
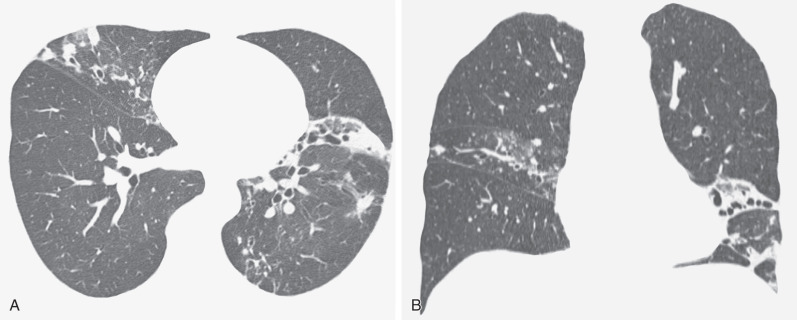
CT findings vary in predictive ability for MAC infection. In a study by Tanaka and coworkers, 26 patients with HRCT appearances suggestive of MAC infection were monitored for 4 years. Half of these individuals subsequently proved to be culture positive for MAC. Similarly, a study by Swensen and colleagues, using the criterion of bronchiectasis with coexistent nodules in the same lobe, found that just greater than 50% of individuals had culture-positive MAC (as opposed to 4% of individuals with CT evidence of bronchiectasis but no lung nodules), thus suggesting a relatively high specificity of 87% and a sensitivity of 80%. In another study the combination of bronchiectasis and nodules in the same lobe, particularly when the middle lobe/lingula was the predominant site of involvement, was highly suggestive of MAC infection. The pathologic correlate of the tree-in-bud pattern in the context of MAC infection is likely to represent exudate in and around the small airways; when present, additional scattered randomly distributed nodules likely represent infective consolidation. Cavitation can be seen in a subset of nodules, with an occasional connection between a terminal or lobular bronchus and the cavity.
CT sometimes reveals mosaic attenuation, reflecting obliterative small airways disease ( Fig. 11.7 ). Whether this is associated with preexisting bronchiectasis or is a direct consequence of MAC infection, as suggested by Kubo and coworkers, is unclear.
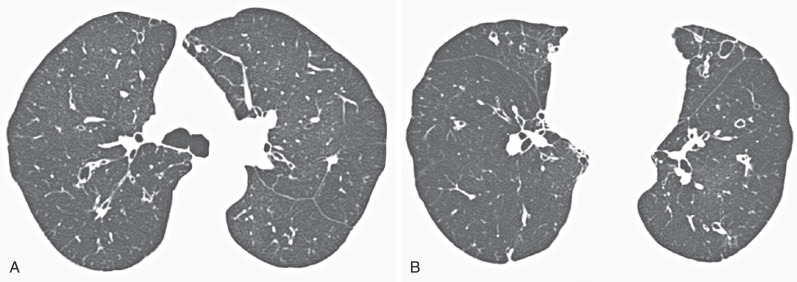
Other occasional features include patchy ground-glass opacification and hilar lymphadenopathy, but these ancillary signs are nonspecific and usually do not occur without the more classic CT findings of MAC infection. Extensive mediastinal lymphadenopathy as a sole manifestation of MAC infection in the absence of HIV infection is extremely rare.
Mycobacterium kansasii
Of all the NTMB, M. kansasii most closely resembles M. tuberculosis, particularly in its responsiveness to conventional antituberculous drugs and its imaging appearances. The exact incidence of M. kansasii infection is unknown, but after MAC it is one of the most common NTMB responsible for pulmonary disease. M. kansasii is particularly prevalent in the southern states of North America and in HIV-positive individuals. Of interest, M. kansasii infection was thought to be relatively uncommon in Korea, but its incidence is now increasing. M. kansasii typically infects middle-aged men rather than elderly women (as in MAC infection). Important risk factors for M. kansasii infection include COPD, alcoholism, pneumoconiosis, neoplasia, previous mycobacterial disease, and other debilitating disease.
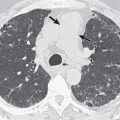
The radiographic appearances of M. kansasii infection are almost always cavitary and involve the upper lobes with coexisting fibrosis and consolidation. The cavities found in M. kansasii infection vary widely in size and wall characteristics, with appearances similar to postprimary pattern of tuberculosis (TB) infection ( Fig. 11.8 ). A CT study by Hollings and colleagues documented that cavities were present in just less than half the patients with M. kansasii infection—the cavities, when present, were confined to the upper lobes; predominantly lower lobe involvement makes M. kansasii infection unlikely. Bronchiectasis, as shown on HRCT, was an invariable feature in the small series ( n = 9) of M. kansasii infection included in the comparative study by Hollings and colleagues. However, of all the NTMB, M. kansasii is the least likely to be responsible for an HRCT appearance characterized by bronchiectasis and bronchiolitis (tree-in-bud pattern) alone. The pattern of M. kansasii infection will be modified by any preexisting pulmonary disease, notably centrilobular emphysema or pulmonary fibrosis ( Fig. 11.9 ).