, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
The most common indications for percutaneous closure of intracardiac communications in adults include atrial septal defect (ASD) and patent foramen ovale (PFO). Other indications for percutaneous closure include ventricular septal defects (VSD), patent ductus arteriosus (PDA), and surgical intracardiac connections, such as shunts (Blalock–Taussig, Glenn, and Fontan) and atrial switch baffles. Transcatheter closures are less invasive than surgical procedures, subject the patient to decreased risk of myocardial ischemia, provide increase patient comfort, and shorten hospitalization.
The most common indications for percutaneous closure of intracardiac communications in adults include atrial septal defect (ASD) and patent foramen ovale (PFO). Other indications for percutaneous closure include ventricular septal defects (VSD), patent ductus arteriosus (PDA), and surgical intracardiac connections, such as shunts (Blalock–Taussig, Glenn, and Fontan) and atrial switch baffles. Transcatheter closures are less invasive than surgical procedures, subject the patient to decreased risk of myocardial ischemia, provide increase patient comfort, and shorten hospitalization.
The various percutaneous closure methods discussed in this section include coils, AMPLATZER occlusion devices (including the duct occluder, septal occluder, muscular VSD occluder, and vascular plug occluder, AGA Medical Corp., Golden Valley, MN), and the CardioSEAL STARFlex septal occluder. The AMPLATZER devices are the most commonly used.
21.1 Percutaneous Closures of Atrial Septal Defect and Patent Foramen Ovale
Transcatheter closure of secundum ASD with an AMPLATZER septal occluder is a common alternative to surgical closure [1–3]. This AMPLATZER device is a self-expanding, self-centering, and recapturable. It has a central waist for defect closure and two disks for device fixation. One disk is positioned in each atrium and the disks are joined by the waist that is positioned across the defect. The size of the device is determined by the diameter of the waist and ranges from 4 to 40 mm (38 mm upper limit in the USA) in diameter (Fig. 21.1). To optimize success, AMPLATZER closure of secundum ASDs requires a sufficient rim of tissue around the septal defect (>4 mm) so that the closure device does not impinge upon the venae cavae or the atrioventricular valves. The CardioSEAL STARFlex septal occluder device (a double umbrella-shaped implant made of a metal framework along with polyester fabric) can be used for smaller defects (Fig. 21.2).

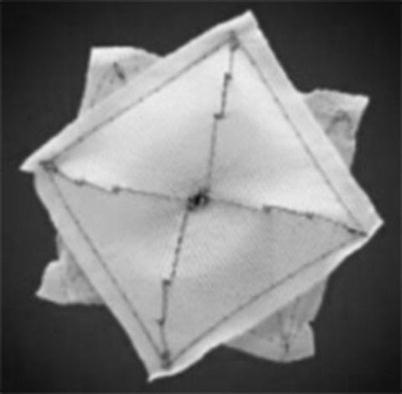

Fig. 21.1
An image of a 38 mm diameter St. Jude MedicalTM AMPLATZERTM septal occluder. The two disks are linked together by a short connecting waist. In order to increase its closing ability, the disks are covered with thin layer of polyester fabric. AMPLATZER and ST. Jude Medical are trademarks of St. Jude Medical, Inc (Photograph reprinted with the kind permission St. Jude MedicalTM. Copyright: 2012. All rights reserved)

Fig. 21.2
A picture of a CardioSEAL device (Nitinol Medical Technologies, Inc., Boston, MA). This device is constructed of a metal framework to which polyester fabric is attached
Contraindications to device closure include sinus venosus and atrioventricular septal defects, secundum ASDs with rim deficiency [4], hypermobile (floppy) interatrial septum (may cause prolapse of the occluder), and a balloon-stretched ASD with a diameter >40 mm (higher risk of device dislodgment) [5]. Since the likelihood of device dislodgment increases if the size of the defect greatly exceeds the waist diameter of the device, the device diameter should be selected according to the balloon-stretched diameter of the ASD to ensure tight stenting of the defect with the prosthesis waist. Computed tomographic (CT) examples of percutaneous closure of secundum ASDs are presented in Figs. 21.3, 21.4, 21.5, 21.6, and 21.7.
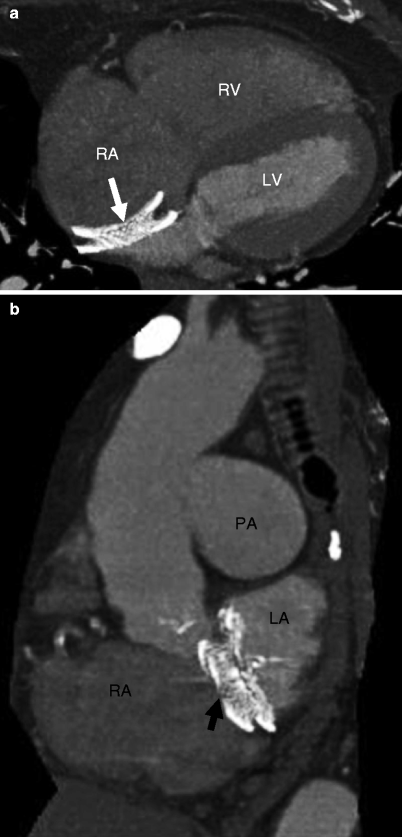
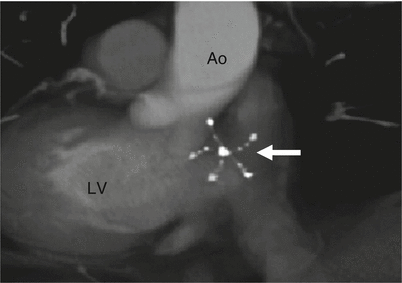
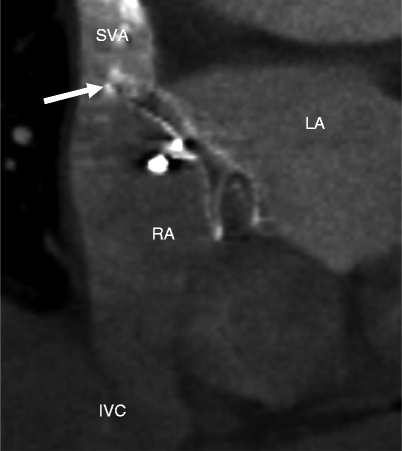

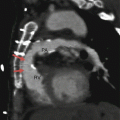
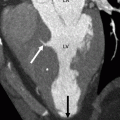
Fig. 21.3
Panels (a) and (b) are axial and sagittal views respectively of an AMPLATZER atrial septal occluder (arrow) demonstrating proper positioning of the device. PA pulmonary artery, RA right atrium, RV right ventricle, LV left ventricle

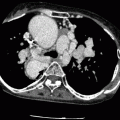
Fig. 21.4
A CardioSEAL occluder. An oblique, sagittal scan showing the septal occluder device (arrow) in its expected position. Ao aorta, LV left ventricle (Reproduced from Johri et al. [6] with permission from BMJ Publishing Group Ltd.)

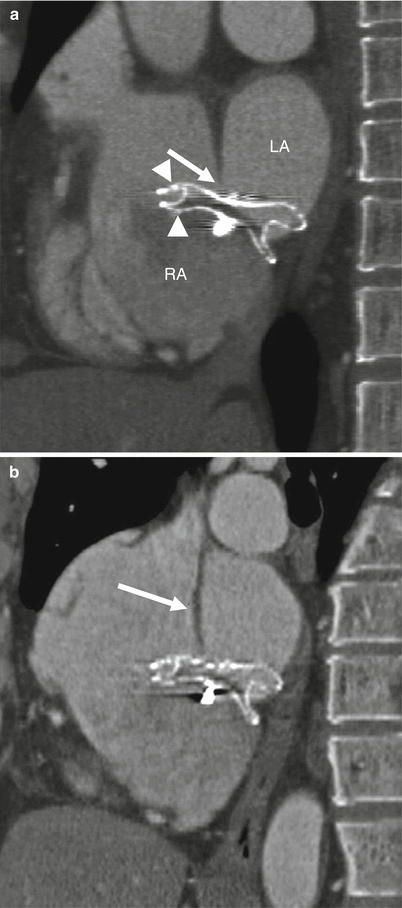
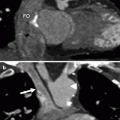
Fig. 21.5
Malpositioned occluder. Minimal device protrusion is noted due to rim deficiency but no residual shunt is present. This oblique, sagittal image shows the upper portion of the AMPLATZER septal occluder device protruding into the right atrium (RA). Both left and right atrial disks (arrow) are in the right atrium. IVC inferior vena cava, LA left atrium, SVC superior vena cava (Reproduced with kind permission of the American Roentgen Ray Society, Leesburg, VA from Lee et al. [7])