Key Points
- •
Near-complete visualization of the entire pleural surface can be obtained by scanning through rib interspaces along the anterior, lateral, and posterior chest wall.
- •
Diagnosis of a pleural effusion requires identification of four standard anatomic structures: lung, diaphragm, fluid collection, and chest wall.
- •
Ultrasound is the most accurate imaging modality for characterization of pleural fluid, and the high resolution of ultrasound allows for identification of pleural-based tumors.
Background
Similar to lung ultrasound described in Chapter 8 , Chapter 9 , pleural ultrasound is predicated on the sonographic characteristics of ribs and lungs. Ribs cast a shadow due to near total reflection of ultrasound waves, preventing visualization of deeper structures. Aerated lungs completely scatter ultrasound waves, generating characteristic artifacts. The pathophysiology of most pleural diseases, except for pneumothorax, includes accumulation of fluid or growth of soft tissue, which are readily visualized with ultrasound.
Technique
Pleural ultrasonography requires a transducer that fits between rib interspaces, typically a sector or phased-array transducer with a frequency of 2–5 MHz (typically 3.5 MHz). Transducers with higher frequencies have better resolution to visualize the pleural surface but have limited penetration (6–9 cm) for many clinical applications.
Orientation of the transducer and screen marker is identical to lung imaging. The screen orientation marker is located in the upper left corner, and the transducer is oriented with the marker pointing cephalad. By maintaining this orientation, the patient’s head will always be toward the left of the screen. Initially, the depth should be maximized to visualize a large area and perform a general assessment of deep structures. After performing a general scan of deep structures, the depth should be reduced and gain adjusted to focus on a specific segment of lung and pleura.
The highly reflective pleura is seen as the first bright line approximately 0.5 cm below the level of the ribs. Sequential evaluation of interspaces can be performed by sliding the transducer longitudinally along the anterior, lateral, and posterior chest wall, and near-complete visualization of the pleura can be obtained. The right diaphragmatic pleura can be viewed transhepatically, but the left diaphragmatic pleura, especially in the absence of pleural fluid, may not be entirely visible due to intervening air-filled lung. The mediastinal pleura cannot be visualized using a transthoracic approach unless a moderate-to-large pleural effusion is present. In general, visceral subcostal pleura is obscured by rib shadowing.
Ultrasound examination of the pleura in ambulatory patients is usually performed in an upright position, while examination of hospitalized or critically ill patients is usually performed in a supine position. A complete exam in a supine patient requires placing the transducer on the posterior axillary line with the ultrasound beam aimed superiorly to avoid missing a layering pleural effusion or dependent or posterior pleural pathology. This maneuver may require additional providers to lift the patient, especially obese patients. In addition, subcutaneous air and excessive fat will prevent visualization or degrade image quality of deep structures. Using firm pressure on the skin and copious gel may reduce some of these limitations.
Pleural Effusion
Ultrasound is well suited for identification and evaluation of fluid because most fluids propagate sound waves without attenuation, allowing for optimal visualization of the borders around and soft tissue contained within fluid-filled spaces. The anechoic nature of most, not all, pleural effusions allows for visualization of pleural lining and compressed, atelectatic lung tissue.
Many studies have demonstrated the usefulness of ultrasound for the identification and characterization of pleural fluid. Pleural effusions as small as 3–5 ml may be detected by ultrasound. Ultrasonography is superior to chest radiography in detecting the presence of pleural effusions and in distinguishing pleural effusions from atelectasis or pleural thickening. Compared to chest computed tomography (CT) scan, pleural ultrasound has 93% sensitivity and specificity for detecting pleural effusions. In addition, the complexity of pleural effusions is better appreciated with ultrasound than with chest CT. This strength makes ultrasound particularly useful in the intensive care unit given the poor performance of portable chest radiographs to detect pleural fluid due to limitations of penetration, magnification, and patient rotation.
Pleural effusions accumulate in dependent areas, but pleural and parenchymal abnormalities are difficult to distinguish on chest radiographs. Opacities on a supine chest radiograph can be caused by pleural effusions, atelectasis, consolidation, or any combination of these processes. Notwithstanding the challenges of acquiring adequate ultrasound images in a supine patient, ultrasound image interpretation is similar in supine vs. sitting patients. Providers should always identify the following three findings to confirm the presence of a pleural effusion:
- 1.
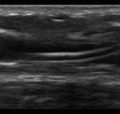
Anatomic boundaries: Identification of the diaphragm, subdiaphragmatic organs (liver or spleen), chest wall, and lung should be clearly differentiated from the pleural effusion ( Figure 10.1 ).
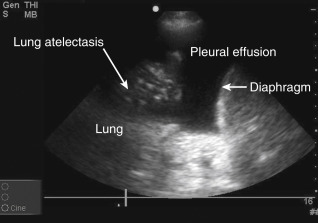
Figure 10.1
Lower lobe compressive atelectasis seen within the anechoic space of a simple pleural effusion.
- 2.
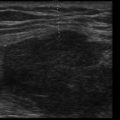
Anechoic space: A relatively anechoic space surrounded by typical anatomic boundaries is a pleural effusion ( Figure 10.2 ).
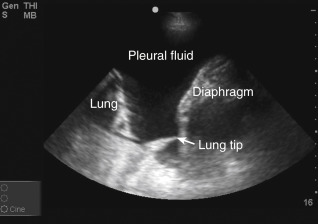
Figure 10.2
Required features to diagnose pleural effusion are present: anechoic space, lung borders seen in far field, curvilinear diaphragm, and chest wall (near field, top of screen). Dynamic movements of diaphragm and lung are not seen in this static image (see ).
- 3.
Dynamic changes: Characteristic changes of the anechoic space should be identified, including (1) typical movement of the lung in a pleural effusion, also called “lung flapping” or the “jellyfish sign,” and (2) diaphragmatic movements (
 ).
).
In a supine patient, providers should begin by scanning along the posterior axillary line. The diaphragm is identified as a hyperechoic, curved structure that moves with respirations, and its location is confirmed by visualizing subdiaphragmatic organs. Pleural fluid is typically less echogenic than the adjacent liver or spleen. Occasionally, a complex pleural effusion will have echogenicity similar to these organs. The inside of the chest wall should not undergo dynamic changes with respiration. Aerated lung may move into the scanning field during the respiratory cycle and block visualization of deeper structures. This has been termed the curtain sign (see Chapter 9, Video 9.2 , and ![]() ). Using M-mode, the pleural surface will generally show a sinusoid sign, indicating dynamic movement of the pleural surface within a fluid-filled space.
). Using M-mode, the pleural surface will generally show a sinusoid sign, indicating dynamic movement of the pleural surface within a fluid-filled space.
Quantifying pleural fluid volume can be calculated with reasonable accuracy using the formula: volume of pleural fluid in milliliters = 20 × (distance between visceral and parietal pleura in millimeters). However, a qualitative assessment of pleural effusion size as small, moderate, or large is sufficient to guide clinical management in most patients.
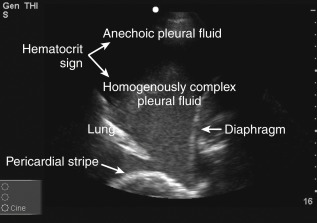
Pleural fluid echogenicity should be described. Transudates are almost always anechoic and should generally not have a heterogeneously echogenic or septated pattern. However, highly cellular transudates may occasionally be homogeneously echogenic ( Figure 10.3 ). Echogenic swirling patterns are highly suggestive of exudates. Exudative effusions often have an echogenic pattern that is not homogeneous, and debris, strands, or septations may be visible. A dynamic sign of pleural fluid is “plankton sign” seen as swirling debris in a pleural effusion during cardiac or respiratory motion. Fibrin strands that are connected to pleural surfaces may be seen during cardiac pulsations or respiratory motion ( Figure 10.4 , ![]() ). In the case of parapneumonic effusions, these findings typically indicate the presence of a complicated parapneumonic effusion or an empyema. Patients with septated effusions visible on ultrasonography require longer hospital stays, longer chest tube drainage, and more often require fibrinolytic therapy or surgery for adequate drainage. Pleural ultrasound is superior to CT scan in visualizing septations within a pleural effusion. Layering of an effusion into two phases of different echogenicities may be observed in hemothorax or empyema and is called “hematocrit sign.”
). In the case of parapneumonic effusions, these findings typically indicate the presence of a complicated parapneumonic effusion or an empyema. Patients with septated effusions visible on ultrasonography require longer hospital stays, longer chest tube drainage, and more often require fibrinolytic therapy or surgery for adequate drainage. Pleural ultrasound is superior to CT scan in visualizing septations within a pleural effusion. Layering of an effusion into two phases of different echogenicities may be observed in hemothorax or empyema and is called “hematocrit sign.”