, Ahmad Ameri1 and Mona Malekzadeh2
(1)
Department of Clinical Oncology, Imam Hossein Educational Hospital, Shahid Beheshti University of Medical Sciences (SBMU), Shahid Madani Street, Tehran, Iran
(2)
Department of Radiotherapy and Oncology, Shohadaye Tajrish Educational Hospital Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran
Radiation pneumonitis (RP), the acute manifestation of radiation-induced lung injury, is one of the main dose-limiting toxicities among patients receiving thoracic radiation therapy. It has been reported to occur in 13–37% of patients receiving definitive external beam radiation therapy for lung cancer [1]. Radiation pneumonitis development following radiation therapy for other cancers actually depends on lung volume, which is included in various irradiated volumes and dose parameters. Some other factors such as smoking, background lung disease, or combined modality treatments are also important risk factors for radiation-induced pneumonitis. The incidence of radiation pneumonitis is lower in Hodgkin disease (3%) [2] and breast cancer patients (1%) [3] compared with lung cancer patients. Diagnosis and indications for therapeutic intervention are in a major controversy that will be discussed in the next few paragraphs.
10.1 Mechanism
The first changes that are induced by radiation are increased vascular permeability and exudation of proteinaceous material in the alveolar space [4]. Capillary endothelial cells are very sensitive to ionizing radiation, and their damages are manifested by detachment of cells from their basement membrane, which is followed by blood flow turbulence and thrombosis formation. Following endothelial cell injury, capillary permeation increases, fibrin-rich exudate leaks into the alveoli, and a hyaline membrane is formed, which impairs gas exchange. Ionizing radiation can also damage alveolar cells, which are manifested by depletion of type I pneumocytes and hyperplasia of type II pneumocytes, in the context of the alveolar epithelium regeneration process [5]. On the other hand, type II pneumocytes are also known to be damaged by radiation therapy, resulting in release of surfactant into the alveolar space and detachment of the pneumocytes from their basement membrane [6]. Later, the alveolar exudate clears, fibroblasts migrate into the alveolar walls, and the alveolar septa are thickened.
10.2 Timing
Although pathologic changes in lung tissue occur in the initial 24–48 h after radiation, those changes are undetectable both clinically and radiologically. Radiation pneumonitis occurs typically within 6 months after a course of radiation with a peak onset at 1–3 months but may be seen as early as 1 week, especially in patients receiving high total dose [7]. In some cases, no symptoms are present, and the diagnosis is made by imaging alone (Fig. 10.1).
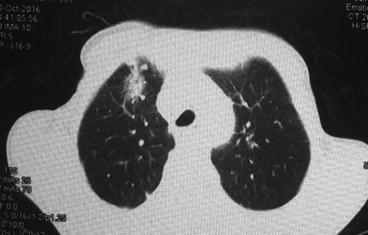

Fig. 10.1
Radiation-induced pneumonitis in a patient with bulky Hodgkin disease at 2 months after mediastinal radiation therapy
10.3 Risk Factors
10.3.1 Volume and Dose Parameters
Nontarget lung dose and irradiated volume should be lowered as much as possible. Different dose and volume parameters (mean lung dose, V5, V10, D15, V20, etc.) have been evaluated to predict probabilities of radiation pneumonitis, but no definitive dose and volume limits have been accepted as an optimal limit or parameter. The percentage of lung volume exceeding 20 Gy (V20), percentage of lung volume exceeding 30 Gy (V30), and mean lung dose (MLD) are the most predictive parameters evaluated in several studies. It has been shown that the risk of radiation pneumonitis is less than 20% when MLD is less than 20 Gy [8], less than 8% when V20 is 31% or lower [9], and less than 6% when V30 is 18% or lower [10]. These parameters are in respect of both lung volumes; however, a significant correlation was recently found between these parameters when ipsilateral lung volume is considered and radiation pneumonitis rates have been reported [11].
10.3.2 Fractionation Schedule
The use of twice-daily fractionation has been shown to reduce the risk of radiation pneumonitis compared with administration of the same total daily dose as a single fraction [12].
Most dose volume parameters have been studied in areas of conventional fractionated radiotherapy of lung cancer, and with increasing use of stereotactic body radiation therapy with a large fraction size for lung cancer, well-designed studies are needed to define new parameters for radiation-induced pneumonitis.
10.3.3 Chemotherapy/Hormone Therapy
The administration of chemotherapy concurrently or before radiation therapy appears to increase the risk of radiation pneumonitis compared to radiation therapy alone [13, 14]. It has been shown that concurrent taxane-based chemoradiation therapy increases the radiation pneumonitis risk more than cisplatin-based chemotherapy regimens [15]. Additionally, chemotherapy administration with certain agents (e.g., taxanes, anthracyclines, gemcitabine, etoposide, or vinorelbine), following radiation therapy, can cause radiation-induced pneumonitis that is an inflammatory reaction within the previously treated radiation field [16].
10.3.4 Smoking
10.3.5 Other Factors
Adequate data are not available for some variables including age, sex, tumor site, Karnofsky performance status, comorbid lung disease, pulmonary function test, and biological markers such as plasma cytokine levels and transforming growth factor beta 1 (TGF-β1).
10.4 Symptoms
Dyspnea is the most common symptom, occurring in as many as 90% of cases. Dyspnea is frequently accompanied by a dry cough, which occurs in about 50–60% of cases. A low-grade fever is occasionally reported, which can be more pronounced in severe cases. Some patients complain of chest pain with breathing, malaise, and weight loss. Sensation of chest fullness usually develops 1–3 months after completion of radiation therapy.
10.5 Diagnosis
Radiation pneumonitis is a diagnosis of exclusion. Physical findings are usually not prominent, but occasionally moist crackles, a pleural friction rub, or evidence of consolidation may be present. There is no specific lab test to predict the development of RP. Some patients might have a mild polymorphonuclear leukocytosis, elevated ESR, serum LDH, and CRP, but these findings are nonspecific.
Chest X-ray and computed tomography are the most commonly used modalities for assessing RP. The most common finding on CXR in early phases is perivascular haziness, which often progresses to patchy alveolar-filling densities [20]. Pleural effusions or atelectases are also sometimes seen. These changes occur shortly after completion of radiation therapy, peak at 6 months, and become stable by 12 months. One of the most characteristic features of radiation pneumonitis and fibrosis is that these radiologic changes are confined to the outlines of the field of radiation (Fig. 10.1). However, the use of oblique beam angles and the development of newer irradiation techniques such as three-dimensional conformal radiation therapy and intensity-modulated radiation therapy can result in an unusual distribution of these findings [21].
Computed tomography (CT) is more sensitive than chest radiograph in detecting subtle lung injury following radiation treatment but is not required to make the diagnosis of RP. The most common findings on CT are ground-glass opacities in the acute phase and traction bronchiectasis, volume loss, and consolidation in the late phase [22].
Pulmonary function test (PFT) in patients with radiation pneumonitis usually reveals a reduction in lung volumes and diffusing capacity of the lungs for carbon monoxide (DLCO), but these changes are nonspecific [23].
Bronchoalveolar lavage and transbronchial biopsy are not useful in the diagnosis of radiation pneumonitis but can be used to exclude other causes of pulmonary infiltrates.
10.6 Scoring
Common Toxicity Criteria for Adverse Effect (CTCAE) (Table 10.1) [24] and Radiation Therapy Oncology Group (RTOG) (Table 10.2) [25] have defined five grades for radiation-induced pneumonitis with some differences.
Table 10.1
CTCAE v4.03 for pneumonitis
Grade 1 | Asymptomatic; clinical or diagnostic observations only; intervention not indicated |
Grade 2 | Symptomatic; medical intervention indicated; limiting instrumental activity of daily life (ADL) |
Grade 3 | Severe symptoms; limiting self-care ADL; oxygen indicated |
Grade 4 | Life-threatening respiratory compromise; urgent intervention indicated (e.g., tracheotomy or intubation) |
Grade 5
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|