Background
Lung cancer is the third most common cancer and the leading cause of cancer-related death in the United States. The most important risk factor for lung cancer is smoking, which results in approximately 85% of all US lung cancer deaths. It is estimated that 224,390 new cases and 158,080 deaths from lung cancer occurred in the United States in 2016. Deaths from lung cancer exceed the combined totals of breast, colorectal, and prostate carcinomas, which are the three next most common causes of cancer death. If mortality rates remain stable, more than 1 million Americans will die of lung cancer in the next 7 years. If there were an effective screening tool for lung cancer that resulted in a 10% mortality reduction, however, an estimated 16,000 lives per year could be saved in the United States alone. There are currently screening programs for breast, colorectal, and prostate carcinomas, all of which have shown a mortality reduction. Recently, screening for lung cancer has begun in the United States based on the findings of the National Lung Screening Trial (NLST).
Conditions for Screening
Numerous conditions must be met to screen effectively for a particular disease. The two primary conditions are (1) an asymptomatic phase of the disease, during which it can be detected, and (2) availability of an effective intervention during the presymptomatic phase that would alter the eventual outcome of the disease.
For the purpose of lung cancer screening, most stage I lung cancers are asymptomatic. It has been estimated that most stage I lung cancers have been present for at least 4 years before diagnosis. Given these estimates, it would seem that lung cancer meets the first criterion for screening.
Previous survival studies after resection of stage I lung cancer have shown survival rates ranging from 62% to 82%. In addition, studies of unresected stage I lung cancer have shown a survival rate of only 4%. These studies would seem to support the second criterion in that intervention at an earlier stage may translate into decreased mortality. Survival and mortality are not equivalent terms, however, and the second condition had not previously been conclusively proven.
History of Lung Cancer Screening
During the 1970s, there were three mass screening trials at Johns Hopkins, Memorial Sloan Kettering, and the Mayo Clinic that used the chest radiograph as the screening modality. In combination, these studies enrolled more than 30,000 men 45 years old or older who were current smokers. In these studies the screening group had more early-stage cancers and increased 5-year survival; however, a mortality reduction from lung cancer in the screened group versus the control group was not shown. Another study done in Czechoslovakia in the 1980s, which involved more than 6000 participants, also failed to show a mortality reduction. Finally, in 1996 extended follow-up of the participants in the Mayo Clinic study showed no mortality difference between the screened and control groups. The inability of the chest radiograph to show a decrease in lung cancer mortality in these studies is why no current organizations recommend using the chest radiograph for lung cancer screening.
History of Computed Tomography Screening
Because the chest radiograph failed to screen effectively for lung cancer, investigators turned to low-dose CT (LDCT) as a potential screening tool. In a seminal study by Kaneko and colleagues, 3457 low-dose spiral CT scans were performed on 1369 men 50 years old or older who had a greater than 20 pack-year smoking history. Each participant was imaged with a chest radiograph and an LDCT scan. The LDCT scan detected 15 lung cancers. Only 4 of the 15 cancers detected on CT were visible on the chest radiograph. CT also was shown to detect smaller cancers. The average diameter of the cancers detected on CT was 16 mm, whereas the average diameter of the cancers detected on chest radiograph was 30 mm. Finally, it was shown that 93% of the CT-detected cancers were stage I. This was much higher than the historic percentage of 20% to 25% potential resectable cancers and gave the impetus for further evaluation of LDCT as a screening modality for lung cancer.
The second important early study that provided encouraging data about the prospects for LDCT screening was the Early Lung Cancer Action Project (ELCAP). This study enrolled 1000 participants who were 60 years old or older and had at least a 10 pack-year smoking history. Participants were imaged with a chest radiograph and a low-dose spiral CT scan. In this study 27 lung cancers were detected on LDCT, and only 7 of these were visible on the chest radiograph. Of the 27 cancers detected on CT, 85% were stage I cancers. These early studies showed CT could identify more cancers and cancers at an earlier stage than was possible using the chest radiograph, which illustrated the potential for CT to be an effective screening tool.
More recently, Henschke and colleagues reported the results of a large collaborative study. These results showed a high incidence of stage I lung cancers (85%). In addition, the group of participants who had clinical stage I lung cancer had an estimated 10-year disease-specific survival rate of 88%. The improved survival was encouraging; however, this study was limited as it did not focus on mortality.
The largest published study on the effectiveness of LDCT for lung cancer screening was the NLST. This double-blind trial enrolled participants who were between 55 and 74 years of age, had a history of cigarette smoking of at least 30 pack-years and, if former smokers, had quit within the previous 15 years. The study excluded persons who had previously received a diagnosis of lung cancer, had undergone chest CT within 18 months before enrollment, had hemoptysis, or had an unexplained weight loss of more than 6.8 kg (15 lb) in the preceding year. A total of 53,454 persons were enrolled. This trial showed a reduction in lung cancer mortality of 20% and a reduction in all-cause mortality of 6.7% (95% confidence interval [CI], 1.2% to 13.6%). Results of this trial have shown a sensitivity of 93.8% and a specificity of 73.4% for the detection of lung cancer on a screening examination. In the NLST the positive predictive value of finding nodules of 4 mm or larger was 3.8%. Modeling has shown that annual screening with LDCT provides the maximal benefit compared with biennial or triennial screening. Benefits were measured as a percentage of early-stage lung cancer detection, number of lung cancer deaths avoided, and total number of life-years gained. Modeling studies have also illustrated the potential issue of overdiagnosis. One study performed for the US Preventive Services Task Force (USPSTF) estimated that 10% to 12% of screen-detected cancer cases would not have been detected in the patient’s lifetime without screening.
In contrast to the NLST, there have been small-scale European trials that have been unable to reproduce the findings of the NLST, concluding that there is no benefit to screening. These trials include the DANTE (Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays) trial and the DLCST (Danish Lung Cancer Screening Trial). However, these were smaller trials ( n = 2472 and 4104, respectively) that may have been underpowered, preventing the study from finding a benefit to screening, should one exist. In addition, the inclusion criteria in the DLCST resulted in younger and healthier participants than in other trials, which may have resulted in bias. The relative risk for all-cause mortality in the DLCST was 1.46 (95% CI, 0.99 to 2.15). This finding highlights the potential harm of screening a young, healthy population.
In Europe the ongoing Dutch-Belgian Lung Cancer Screening Trial (NELSON [Nederlands-Leuvens Longkanker Screenings ONderzoek]) is investigating whether screening with LDCT can reduce lung cancer mortality by at least 25% compared with no screening at 10 years of follow-up for individuals aged 50 to 75 years who had a smoking history of greater than or equal to 15 cigarettes per day for greater than or equal to 25 years or greater than or equal to 10 cigarettes for greater than or equal to 30 years, and who were still smoking or had quit less than or equal to 10 years ago. Since the start of the NELSON study in 2003, more than 7000 participants were randomized to CT screening. Results from the NELSON Trial are anticipated soon. The findings of this study will be a valuable addition to the current data on LDCT for lung cancer screening.
Screening Programs and Reimbursement
Using data from NLST and modeling studies, the USPSTF determined that annual LDCT screening provides net benefit to patients aged 55 to 80 years at high risk for lung cancer. Currently, the USPSTF recommends annual screening for lung cancer with LDCT in adults aged 55 to 80 years who have a 30 pack-year smoking history and are currently smoking or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. This is a category B recommendation. The implications for this USPSTF recommendation are that insurance companies in the United States are now required to cover lung cancer screening for their enrollees.
More recently, the Centers for Medicare and Medicaid Services (CMS) has approved lung cancer screening for participants enrolled in Medicare. The age group covered by CMS differs slightly from the group covered by the USPSTF recommendations. CMS also has several reporting requirements that must be met in order for the lung cancer screening episode to be reimbursed. These include shared decision making, reporting of the scanning protocol and findings, and the development of a registry that can track patient outcomes after screening. To help with these requirements, the American College of Radiology (ACR) has developed scanning and reporting protocols for lung cancer screening and has also developed a registry that can be used. As specified by the ACR–Society of Thoracic Radiology (STR) Practice Parameter for the Performance and Reporting of Lung Cancer Screening Thoracic Computed Tomography, a CT scanner should meet or exceed the following capabilities: (1) gantry rotation times: less than or equal to 0.5 seconds, (2) slice thickness: less than or equal to 2.5 mm (≤1.0 mm is preferred), and (3) preferably greater than or equal to 16 detector rows. In addition, the CT scanner and/or the viewing platform should be capable of generating maximum-intensity projection and multiplanar reconstruction images. The low-dose screening technique should be set to yield a computed tomography dose index volume of less than 3 mGy for a standard-sized patient (typical average radiation dose of 1.5 mSv). It should be reduced for smaller-sized patients and increased for larger-sized patients.
Lung Computed Tomography Screening Reporting and Data System (Lung-RADS)
To assist radiologists with reporting the findings from lung cancer screening examinations, the ACR has developed Lung-RADS, which allows standardized impressions and recommendations to be generated based on the findings on the screening CT examination. The most updated version of Lung-RADS can be found on the ACR website. The primary drivers for determination of the impression and recommendation are the size and attenuation of the nodule. This differs slightly from the NLST, which only took into account the size of the nodule when making recommendations.
Studies by Henschke and colleagues and Midthun and colleagues have shown that for nodules less than 5 mm the likelihood of malignancy is less than 1%, even in a high-risk screening population. In addition, even when these tiny nodules were shown to be lung cancer, most of these were still stage I cancers. Although the nodules would still need to be followed, a less aggressive follow-up of these smallest nodules would be possible according to these results. Using these and other studies, Lung-RADS changed the recommended size for a positive study that would require shorter-term follow-up from 4 mm (as in the NLST) for a solid nodule to 6 mm.
The attenuation characteristics of the nodules are broken down into solid, part-solid, and pure ground-glass. If nodules are part-solid, an additional measurement of the solid component of the nodule is required to appropriately guide the recommendation for further workup. The attenuation of the nodule also influences the recommendations for the size of the nodule as solid nodules have different recommendations based on size compared with part-solid and ground-glass nodules. Part-solid nodules also take into consideration the size of the solid component when making recommendations for further workup. Margins of the nodule and associated findings can also be taken into account when making the recommendation for further workup.
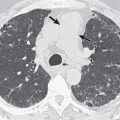
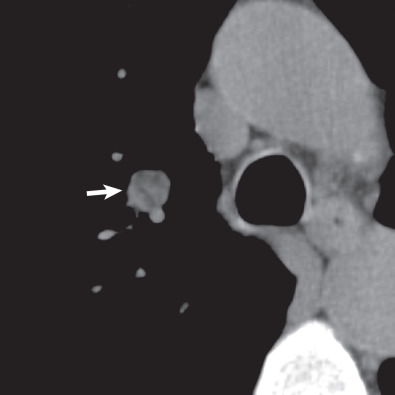
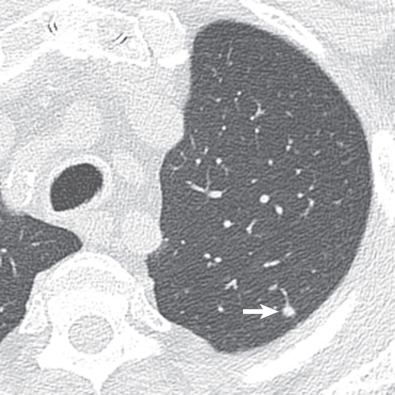
There are five main Lung-RADS categories plus additional modifiers (see the most up-to-date version of Lung-RADS at www.acr.org ). These include categories 1 (negative; Figs. 16.1 and 16.2 ) and 2 (benign appearing; Figs. 16.3 and 16.4 ), which correspond to a negative screening. Categories 3 (probably benign) ( Fig. 16.5 ) and 4 (suspicious) correspond to a positive screening. Category 4 is further divided into 4A ( Fig. 16.6 ), 4B ( Fig. 16.7 ), and 4X ( Fig. 16.8 ). Category 0 is given to an examination that is considered incomplete. All nodules should be measured on lung windows and reported as the average diameter rounded to the nearest whole number. A negative screening result assumes that reevaluation will occur at the next annual screening, whereas a positive screening result means that additional evaluation is recommended before the next annual screening. This evaluation often involves a short-term follow-up diagnostic CT that can be performed with a low dose. Category 3 or 4A nodules that are unchanged on the interval CT scan should be recoded as category 2 and the patient returned to annual screening. Nodules with additional worrisome features (such as spiculation) or imaging findings that increase suspicion for cancer (such as enlarged lymph nodes) can qualify as category 4X (see Fig. 16.8 ), even if the nodule would have been classified as a category 3 nodule based on size. The overall Lung-RADS screening category is determined by the nodule with the highest individual Lung-RADS score.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree