Chapter 11 Thoracic Aortic Disease
INTRODUCTION
Just as a primary cardiac problem can affect other organ systems, systemic disease or an abnormality in another organ system can secondarily cause cardiac dysfunction. Deciding which is the primary abnormality can pose a diagnostic dilemma. For example, in a patient with aortic regurgitation, is the cause intrinsic aortic valve disease with secondary aortic dilatation, or is it an aortic aneurysm with dilatation of the aortic annulus that prevents coaptation of the aortic leaflets? It is generally better to do noninvasive tests first—such as chest radiography, computed tomography (CT), echocardiography, and magnetic resonance imaging (MRI)—before invasive angiography to examine the aorta and the aortic root. These diseases require imaging of the thoracic aorta to size the aortic annulus and to detect stenoses or enlargement of the aorta (in addition to aortic valvular stenosis), fistulas to the cardiac chambers or systemic vessels, and intrinsic aortic wall abnormalities.
AORTIC ANATOMY AND SIZE
The size of the aorta is often critical for diagnosing aortic disease. Several measurements are useful in identifying the upper range of normal. On the frontal chest film the distance between the left border of the trachea and the lateral border of the aortic arch is always less than 4 cm in adults and usually less than 3 cm in those younger than 30 years of age. On an aortogram or tomographic scan of the ascending aorta, the normal diameter should be less than 4 cm (Table 11-1). Longitudinal enlargement is more difficult to quantitate but is manifest by tortuosity, occasional kinking or buckling, and displacement into the adjacent lung or mediastinum.
TABLE 11-1 Size of the normal adult thoracic aorta
| Mean (cm) | Upper Limit of Normal* (cm) | |
|---|---|---|
| Aortic root | 3.7 | 4.0 |
| Ascending aorta | 3.2 | 3.7 |
| Descending aorta | 2.5 | 2.8 |
* Two standard deviations above the mean.
From Aronberg DJ, Glazer HS, Madsen K, et al.: Normal thoracic aortic diameters by computed tomography, J Comput Assist Tomogr 8:247-250, 1984; Drexler M, Erbel R, Muller U, et al.: Measurement of intracardiac dimensions and structures in normal young adult subjects by transesophageal echocardiography, Am J Cardiol 65:1491-1496, 1990.
The diameter of the normal adult aorta has a wide range that gradually increases with age (Fig. 11-1).
AORTIC ANEURYSMS
Wall Mechanics
Wall stress or tension in any blood vessel is directly related to blood pressure and vessel radius. This finding is supported by Laplace’s law, whereby wall tension is proportional to the radius of the vessel at a given blood pressure. The larger the vessel radius, the greater the wall stress. Areas of wall weakness can and will expand, resulting in an even greater wall stress and an increase risk of rupture. Wall stress in aneurysmal blood vessels, however, is not uniform compared with adjacent nonaneurysmal vessel wall. The abrupt change in vessel radius in an aneurysmal segment results in significant wall tension differences throughout the aneurysm.
Size and Types
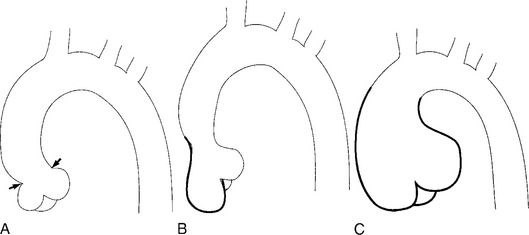
There are a number of definitions of thoracic aneurysm, but most are keyed on the size at which there is potential for rupture (Fig. 11-2). An aortic diameter greater than 1.5 times normal is a commonly accepted definition of aneurysm. For practical purposes, aneurysms in the ascending aorta are greater than 5 cm, and those in the descending aorta are greater than 4 cm.
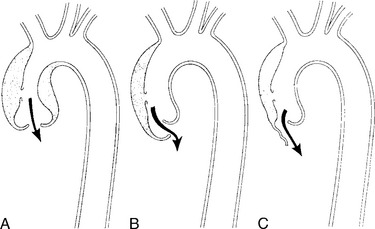
True aneurysms have all elements of the aorta incorporated into the wall of the aneurysm. The most common true aneurysm is a fusiform aneurysm that involves the entire circumference of the aorta. A saccular aneurysm is an eccentric dilatation that involves one side of the aorta (Fig. 11-3). For example, most infected aneurysms are saccular. It can occur in a short segment or can involve the entire aorta. A vast majority of asymmetric or saccular aneurysms fall under the category of false or pseudoaneurysms. False aneurysms have a perforation into the intima and media. An example is an aortic transection from trauma.
A radiologic description of the aneurysm includes the location and extent, the type, and in conjunction with the clinical history, the cause. Box 11-1 lists the common associations, but many aneurysms fall into several categories. Atherosclerotic aneurysms are usually fusiform and occur in the inferior parts of the aorta. Infected aneurysms may be either true or false. Syphilitic aneurysms can be either saccular or fusiform. The list is not inclusive but serves as a starting point for diagnosis and management.
Chest Film Findings
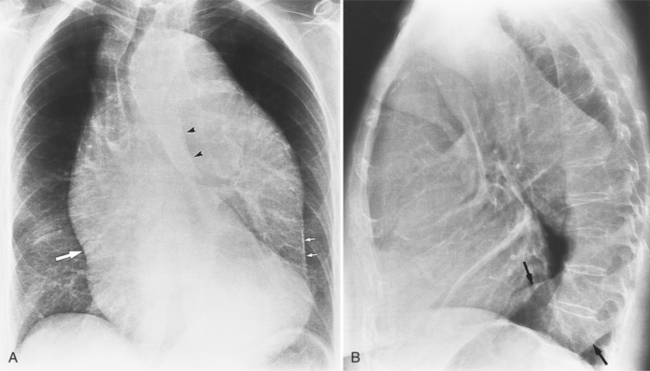
The chest film is one of the first examinations obtained when thoracic aortic aneurysm, dissection, or transection is suspected, even though its sensitivity and specificity for these diagnoses are widely variable. The purpose of the chest film is to assess the size of the aorta and to identify a rupture. A valuable use of the chest film is to follow the mediastinal contours over a time interval to detect an increasing mediastinal width (Box 11-2). Tracheal or bronchial compression can be suspected when these structures are extrinsically deviated (Fig. 11-4). Compression of a pulmonary artery is recognized by unilateral pulmonary oligemia. For most of its thoracic course, the lesser curvature of the aorta is not visible on the chest film so the only signs of aortic enlargement are those of the greater curvature displacing adjacent structures. Other signs appear when the aorta has ruptured into the mediastinum. Unilateral pleural fluid or pericardial fluid usually indicates impending exsanguination or cardiac tamponade. Rupture into the mediastinum is initially constrained by the tissue in the mediastinum and pleural compartments. The chest film signs of aortic rupture are those of mediastinal hemorrhage (Box 11-3).
ACQUIRED DISEASES
Aortic Dissection
Although Morgagni recognized aortic dissection at necropsy over 200 years ago, even today it may be difficult to diagnose, and it certainly remains a therapeutic enigma. Most aortic dissections, or dissecting hematomas, at least in their early stages, are not aneurysms because they are not a localized enlargement of the aorta. The term dissecting aneurysm should be reserved for those cases in which the aorta (usually the false channel) is actually dilated. The clinical picture of a person with abrupt, severe chest pain associated with a loss of one or more peripheral pulses strongly suggests dissection; however, atypical onset of myocardial infarction, pulmonary or systemic emboli, musculoskeletal syndromes, and other chest pain syndromes may mimic the presence of aortic dissection, and therefore aortic imaging is mandatory for a definitive diagnosis. Similarly, a small percentage of dissections are “silent,” occurring without pain, and are discovered only from an abnormal chest film. In these cases, other types of thoracic aneurysm, penetrating aortic ulcer, or nonvascular mediastinal disease must be distinguished from aortic dissection.
Complications
Aortic rupture into the pericardium, pleural space, or mediastinum can be suggested on the plain chest film by a large heart diameter, pleural fluid, and a wide mediastinum. This heralds the need for immediate pericardiocentesis and other cardiopulmonary supportive measures. A dissection can partially or completely occlude a branch of the aorta by compression of the true channel by the false channel or by adjacent compression from an intimal flap. Any artery arising from the aorta can be occluded, but the right coronary artery and the three arch vessels are commonly affected. Surgical treatment aims at preventing retrograde tear into the heart and pericardium by resecting the segment that contains the entry tear. An interposition graft is then inserted, collapsing the false channel distally. Dissections in the aortic root are treated with a composite graft with the prosthetic aortic valve sewn to the graft and the coronary arteries replanted into the sides of the graft.
Plain Film Findings
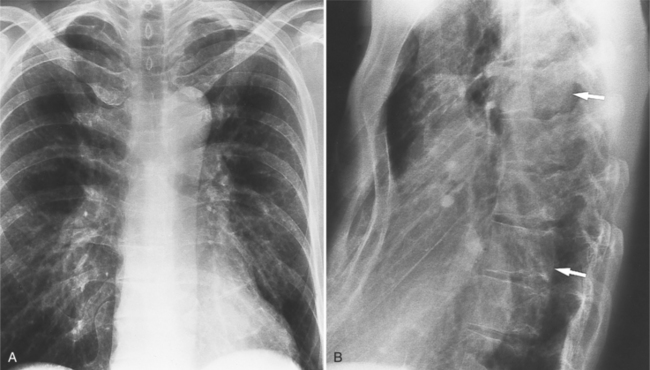
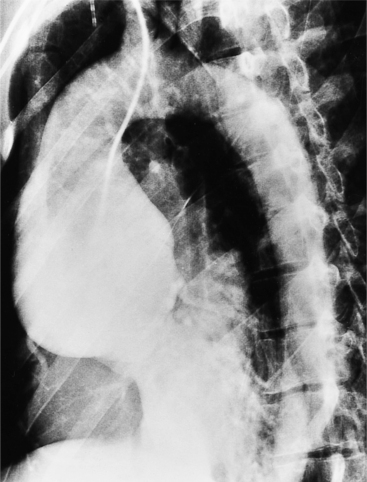
The plain film findings of dissection are indirect but can suggest the need for further evaluation (Fig. 11-5). An abnormally wide mediastinum, separation of calcium from the wall of the aortic arch, a left apical pleural cap, pleural fluid, and displacement of the trachea and esophagus from the midline are important characteristics of a thoracic aortic abnormality. However, the chest film findings are insensitive for the detection of aortic dissection: Nearly one fifth of patients with dissection have normal chest films.
Tomographic Imaging
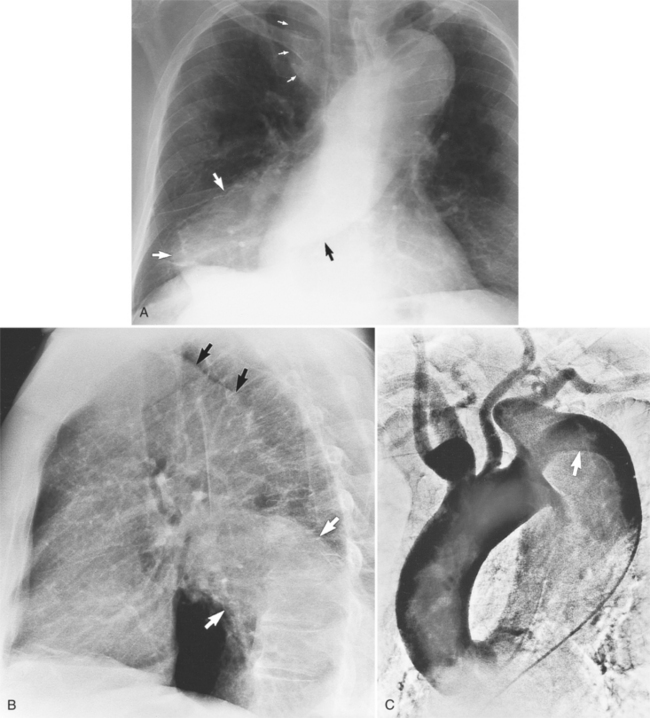
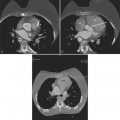
Computed tomography with intravenous contrast, magnetic resonance imaging (MRI), and echocardiography all have excellent sensitivity and specificity for detecting aortic dissection but each has limitations specific to its technology. Optimal computed tomography (CT) imaging is with intravenous contrast on a spiral scanner (Figures 11-6, 11-7). Spiral CT requires a correctly timed bolus of contrast media and has streak artifacts from the pulsating aortic wall that can mimic the intimal flap. Multidetector CT scanners with three-dimensional postprocessing reconstructions can rival the accuracy of catheter angiography.
Magnetic Resonance Imaging
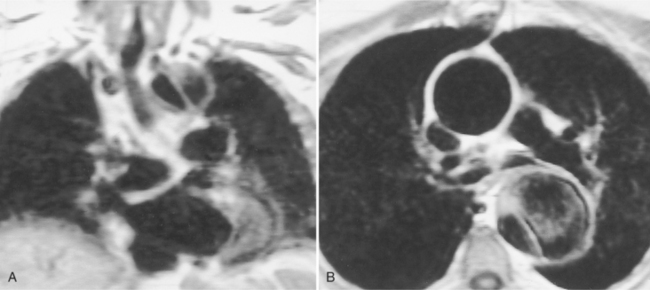
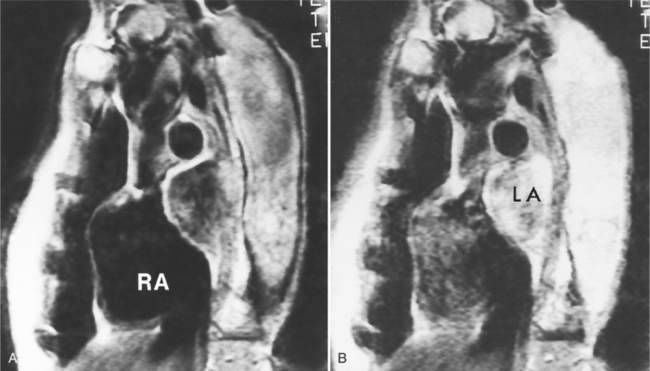
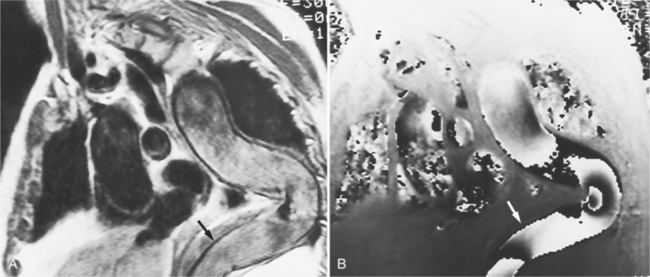
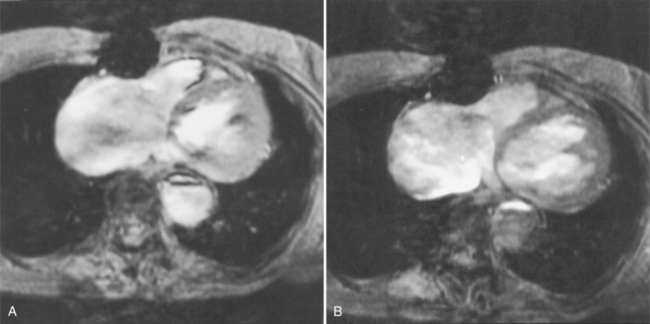
Because of slow blood flow, clotted false channels, and occasionally the twisted shape of the intimal flap, aortic dissection can be difficult to distinguish from other types of aneurysm and aortitis (Fig. 11-8). MRI is particularly useful in these situations because different pulse sequences and reconstruction techniques can be exploited to produce a distinction between flowing blood and static tissue. Spin echo sequences producing “black blood” images easily show the intimal flap when there is moderate flow in both channels. But in regions of slowly flowing blood, the signal in that region may be similar to tissue in the aortic wall and adjacent mediastinum. A number of techniques exist that can image slow velocity differences and separate nonmoving and clotted blood in the false channel from slow-moving blood. Even echocardiographic rephasing, the fortuitous occurrence of velocity compensation in the second echocardiogram, is recognized as a higher signal intensity in the second echocardiogram in regions of slowly flowing blood (Fig. 11-9). A caveat is that failure of the signal intensity on the second echocardiogram to be greater than the first echocardiographic image is often observed in regions of complex velocity changes such as those that occur in vortices and eddies around bends in the aorta.
One of the most sensitive ways to make the distinction between thrombus and slowly flowing blood is to reconstruct the original data as a phase image. All MRIs are generated as complex numbers, which are typically reconstructed as magnitude images. However, the same data can be displayed as a phase image, which then becomes a picture of the velocity of the tissue within each pixel. The phase image needs to be interpreted with the magnitude image to identify the area of concern where a thrombosed channel may be present. Changes in signal intensity, including alternating white to black phase breaks in the phase image, indicate flowing blood (Fig. 11-10).
Another strategy that can be quite helpful is to obtain a gradient echo cine study through the area that has questionable flow in the standard spin echo image. With velocity compensation, the gradient echo pulse sequence should be obtained at only one slice to avoid the inflow of partly saturated spins from a neighboring slice (Fig. 11-11).
Unlike the other techniques, transesophageal echocardiography (TEE) can be carried out at the bedside. The major advantage over the other techniques is the quantitation of aortic regurgitation with color-flow Doppler. Its disadvantage is that the aortic arch cannot be completely imaged with either the transthoracic or transesophageal technique.
Catheter Approach
The site of catheterization will depend on which pulses are present. If no extremity pulses are felt, a pulmonary angiogram with delayed follow-through may show the dissection. However, a general principle is that angiography should be performed with an injection as close to the abnormality as possible, and therefore a femoral, axillary, or brachial arterial approach is preferred. Because the left iliac artery is most frequently involved with a dissection, the preferred route is a percutaneous transfemoral approach from the right side.
Signs of Dissection
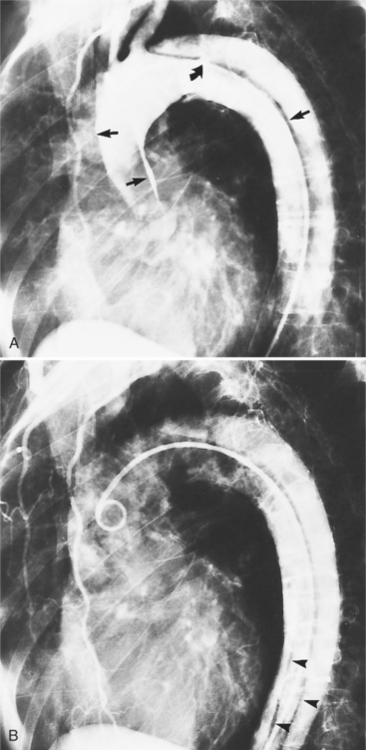
The intimal flap, a lucency several millimeters thick outlined by contrast on both sides, is the hallmark of dissection. You can often identify the actual entry from one channel into another or into multiple channels and the subsequent flow of blood in either an antegrade or retrograde direction. This intimal tear may extend only a few centimeters (Fig. 11-12) or may extend the entire length of the aorta, even into peripheral vessels (Fig. 11-13). The leaflets of the aortic valve, particularly with annuloaortic ectasia or Marfan syndrome, may be effaced and appear as a lucency; the large aortic leaflets can be difficult to distinguish from a true intimal tear in the aortic root, particularly if large-film technique is used. Cine angiography usually resolves this problem. True tears may be further differentiated by their origin above the sinotubular ridge and extension backward into the valve.
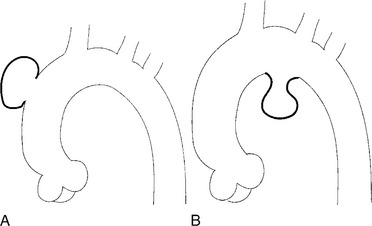
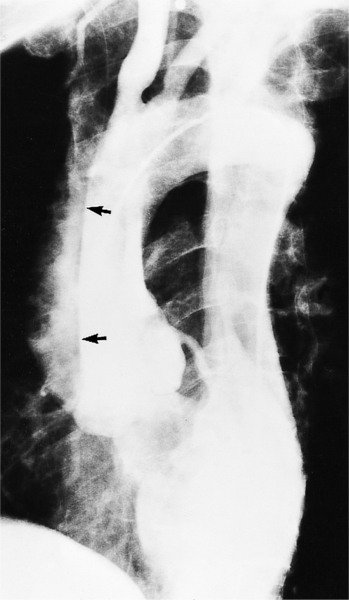
An ulcerlike projection from the aorta may represent an early sign of dissection, although other types of aneurysms, including those caused by infection and penetrating atherosclerotic ulcer, may be associated with this finding. The base of the ulcer represents the defect in the intima leading to a thrombosed false channel (see Figure 11-13). In the descending aorta where side branches originate, the ulcer may be an occlusion or detachment of the intima from an intercostal artery.
The false channel may not opacify during angiography and, therefore, may present as a thick wall (usually >1 cm) along the greater curvature of the aorta (Fig. 11-14). An eccentric wall thickness greater than 1 cm is unusual in a clotted atherosclerotic or syphilitic aneurysm or aortitis. An unopacified channel may occasionally be seen if the injection was made proximal to the entry tear and the distal dissection has extended in a retrograde direction. Before the diagnosis of a thrombosed false channel is made, an injection should be made with the catheter at the level of the diaphragm to search for retrograde flow from a distant entry site. The thrombosed false channel has been called a “healed” dissection and is less liable to rupture late. Initially, the true channel is frequently smaller than the false channel, although either may enlarge to greater than the normal aortic width, then justifying the term dissecting aneurysm.
Aortic regurgitation may result from three mechanisms (Fig. 11-15):
In addition to occluding or transecting any vessels from the aorta, a dissection may compromise other mediastinal vessels. The expanding hematoma of the false channel may compress the right pulmonary artery. Similarly, it may both displace and compress the superior vena cava. On the left side of the mediastinum, because the false channel extends posterolaterally, the pulmonary veins are occasionally compressed. If this abnormality leads to reduced flow through the left lung, then a dissection may be confused with pulmonary embolism on a ventilationperfusion lung scan.
Annuloaortic Ectasia and Marfan Syndrome
Root Enlargement
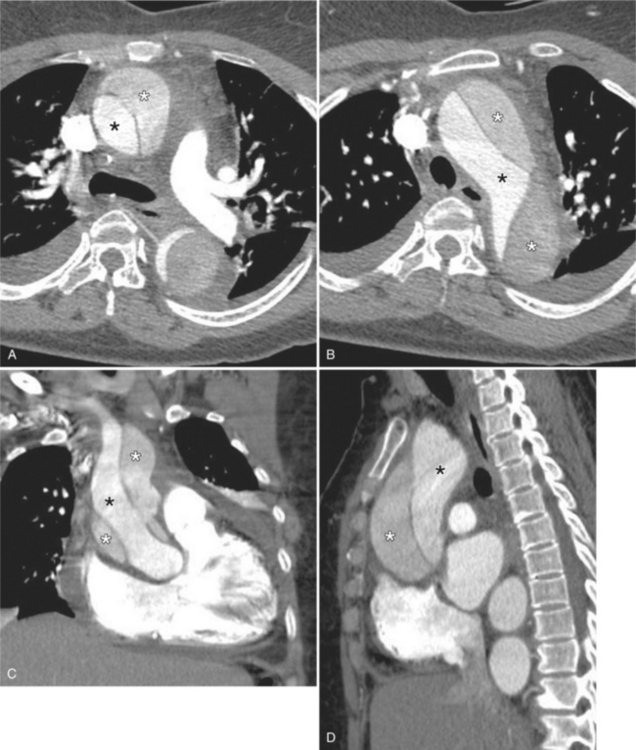
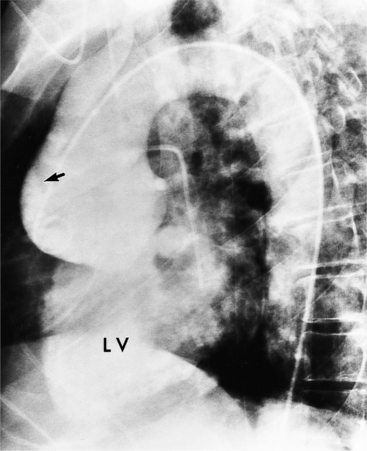
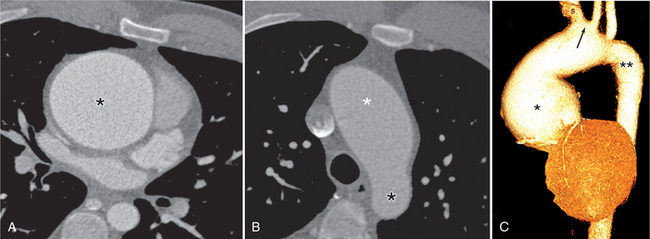
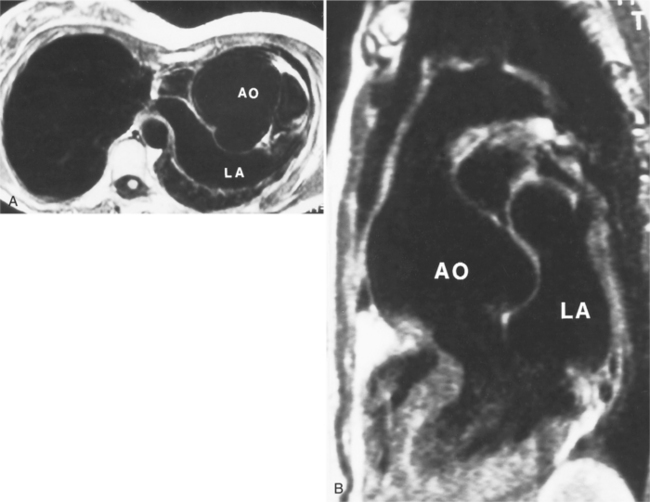
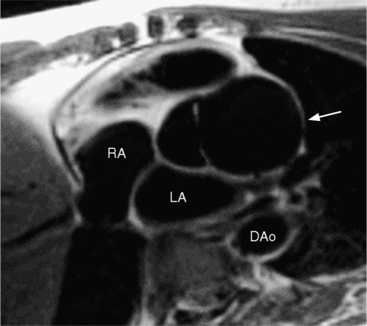
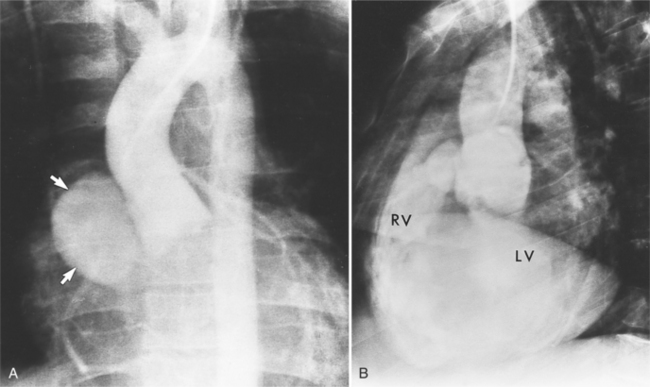
In Marfan syndrome, the sentinel vascular abnormalities in the aorta are aortic regurgitation, fusiform dilatation of the aorta, and dissection. These are represented pathologically by cystic medial necrosis. Degeneration of the aortic media leads to dilatation of the annulus. The aortic leaflets are spread apart and aortic regurgitation ensues. However, patients who do not have other features of Marfan syndrome may have annuloaortic ectasia. The term annuloaortic ectasia describes pear-shaped dilatation of the sinuses of Valsalva and the proximal aorta. Persons without the Marfan syndrome but with annuloaortic ectasia are usually male (by a 2:1 ratio) and are typically first seen after age 40 (Fig. 11-19). Those with Marfan syndrome have clinical symptoms at a much younger age. The aorta in homocystinuria may have an identical appearance.
Marfan Syndrome Presentation
In contrast to isolated annuloaortic ectasia, Marfan syndrome is a generalized disorder of connective tissue demonstrating autosomal dominant inheritance and manifested by cardiovascular, ocular, and skeletal abnormalities. Involvement of the cardiovascular system occurs in more than half of affected adults. Dilatation of the aortic annulus and ascending aorta are usually the first abnormal signs. Later, aortic regurgitation appears, which may ultimately cause left ventricular failure. Aortic dissection is a common complication and is frequently the cause of death (Fig. 11-20). Mitral regurgitation is the most common cardiac abnormality in children and is the result of redundant, elongated chordae tendineae and overlarge leaflets that produce prolapse of the leaflets into the left atrium. Calcification of the mitral annulus in children may occasionally be seen.
Radiologic Techniques
Many of the clues to the diagnosis of Marfan syndrome are frequently visible on the chest film (Fig. 11-21). On the frontal film, the thoracic cage appears large and elongated with large-volume lungs. The heart may be shifted to the left from a narrow anteroposterior thoracic diameter. In normal young adults less than 20 years of age, the aorta should be inapparent. In contrast, aortic elongation and ectasia in this age group are common signs of Marfan syndrome. Cardiomegaly is usually nonspecific and may reflect only the pectus excavatum, but aortic regurgitation from annuloaortic ectasia and mitral regurgitation from prolapsing mitral leaflets are common conditions that pathologically enlarge the heart. On the lateral film, a pectus excavatum is frequently identified as well as a narrow thoracic diameter.
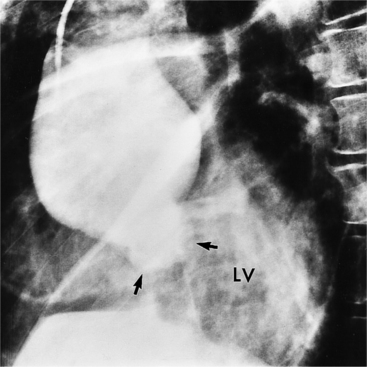
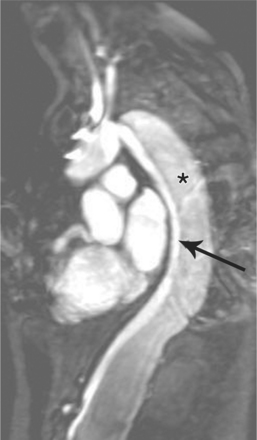
Marfan patients without symptoms are easily observed with serial MRI every 6 to 12 months. Surgical referral is usually undertaken if a previously stable aortic aneurysm begins to enlarge or if the aortic arch and descending aorta exceed a diameter of 5 cm. MRI allows detection of the onset of annuloaortic ectasia (Fig. 11-22) with dilatation of the aortic root and ascending aorta, and visualization of a dissection. Aortic regurgitation can be observed and quantified with velocity-encoded pulse sequences. Observations on the aortic root and quantification of aortic regurgitation can also be made by echocardiography.
Aortography is usually reserved for urgent clinical situations in which noninvasive imaging was inconclusive. Some surgeons request coronary angiography to evaluate whether a dissection extends near or into the coronary arteries. Occasionally, aortography can identify an entry site of a dissection that is not apparent on other methods (Fig. 11-23).
Sinus of Valsalva Aneurysms
Etiology
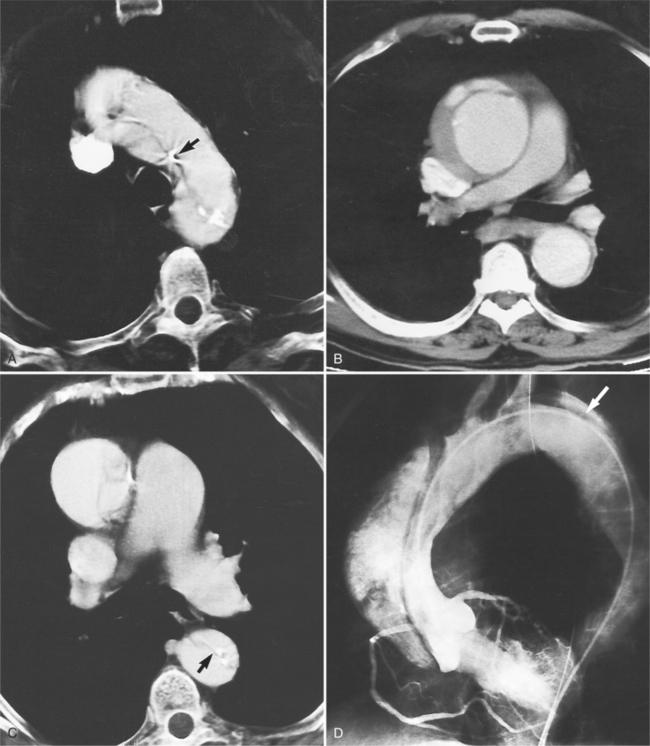
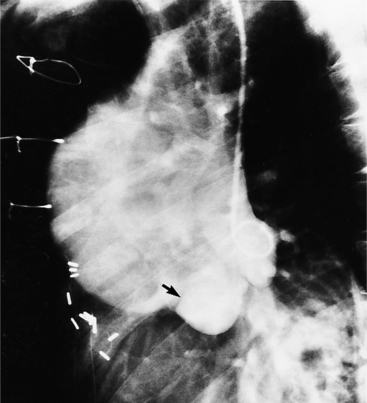
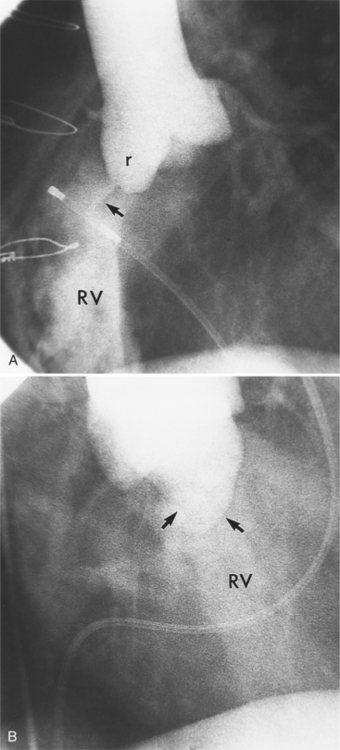
An outline of sinus of Valsalva aneurysms is presented in Box 11-4. Discrete aneurysms that involve a single sinus are usually congenital (Fig. 11-24), although rarely dilatation of two or all three sinuses may also be congenital. These are generally less than 4 cm in diameter and involve mainly the right sinus. The tissue in the aortic annulus adjacent to the leaflet histologically has sparse fibroelastic elements and grossly may have fenestrations through the cusp. A sinus of Valsalva aneurysm can develop as a consequence of a ventricular septal defect. One of the ways a ventricular septal defect can close spontaneously is to form fibrous tissue around its edges. As the membranous ventricular septal defect becomes smaller, the adjacent leaflet of the aortic valve is pulled inferiorly into the defect. The clinical consequence of the developing leaflet prolapse is that the left-to-right shunt through the ventricular septal defect is transformed to that of aortic regurgitation.
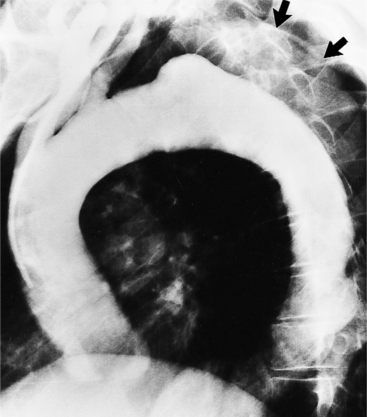
Because the sinuses of Valsalva lie completely within the cardiac silhouette (Fig. 11-25), the discrete type of aneurysm is not visible on the plain chest film. If the ascending aorta is also dilated, the right side of the mediastinum will have the characteristic convexity of the aorta as it extends into the adjacent lung.
Calcification
Calcification of the sinuses of Valsalva above the aortic leaflets is rare. If there is also extensive aortic calcification, this indicates syphilitic aortitis. Mild calcification of the nondilated sinuses and flecks in the ascending aorta suggest the presence of type II hyperlipoproteinemia. Rarely, congenital or nonsyphilitic aneurysms in the aortic root may calcify.
Complications
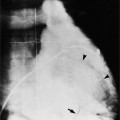
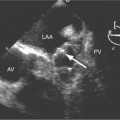
Aortic regurgitation is the main complication of progressive dilatation of the aortic annulus and the resultant lack of coaptation of the leaflets. Any type of sinus of Valsalva aneurysm can rupture into an adjacent structure. The onset is abrupt with severe aortic regurgitation or a torrential left-to-right shunt. Most sinus aneurysms rupture into the right sinus; they perforate anteriorly into the right ventricular outflow tract, dissect into the ventricular septum, or perforate posteriorly in the right atrium. Aneurysms of the noncoronary sinus rupture into the right atrium. Rupture of the left sinus into the left atrial appendage is extremely rare. When an aneurysm ruptures, aortography shows contrast medium entering the cardiac chamber and opacifying downstream structures on subsequent films (Fig. 11-26). Both a left ventriculogram and an aortogram may be necessary to distinguish a ventricular septal defect with aortic regurgitation from a ruptured sinus of Valsalva aneurysm. The contrast in the right ventricle from an aortogram could have passed into the left ventricle from aortic regurgitation and then across a ventricular septal defect, or it could have flowed directly from the aorta through the rupture into the right ventricle.
Aortitis
A number of clinical syndromes have vasculitis that involves the aorta. Most of these diseases are either associated with or caused by immune complexes deposited in the vessel wall. Intimal proliferation and fibrosis, degeneration of the elastic fibers, round cell infiltration, and occasionally giant cells usually allow a specific histologic diagnosis. The gross changes, except in Takayasu disease, are far less specific, regrettably so because these are the features seen on angiography and cross-sectional imaging. Aortitis produces aneurysms in many portions of the aorta and its related branches. They are usually fusiform but occasionally saccular. Takayasu disease is the only aortitis that produces stenoses in the thoracic aorta. Significant stenoses in the aortic arch are well known in the acquired disease: aortic dissection, false aneurysms from laceration of the aortic arch after a motor vehicle accident, infected aortic aneurysms with abscess formation, Behçet’s disease, and rarely atherosclerotic and syphilitic disease.