The wide variety of tracheal diseases includes both benign and malignant conditions that may manifest as tracheal masses, focal or diffuse tracheal thickening, or tracheal calcification. Tracheal tumors, inflammatory conditions involving the trachea, infections, and posttraumatic or iatrogenic injuries are among the variety of tracheal pathologies that can be characterized at imaging. Structural abnormalities of the trachea include acquired or congenital tracheal stenosis, tracheomalacia, and tracheomegaly.
Although a subset of tracheal diseases may be first suspected at chest radiography, computed tomography (CT) is requisite for characterizing the extent and morphology of tracheal pathology. Volumetric acquisitions with multiplanar and three-dimensional (3D) reformats can accurately depict tracheal abnormalities, and the use of inspiratory and expiratory imaging is typically used to assess for tracheomalacia and potential air-trapping within the lungs.
Tracheal Neoplasms
Etiology
A majority of primary tracheal neoplasms in adults are malignant. The most common cell type is squamous cell carcinoma, which is associated with cigarette smoking. The second most common cell type is adenoid cystic carcinoma, which is not smoking related. The most common benign tracheal neoplasm is squamous cell papilloma, which may be solitary or multiple. Solitary papillomas have an association with cigarette smoking, whereas multiple papillomas (also referred to as papillomatosis ) are linked to infection by the human papillomavirus. A variety of other benign and malignant neoplasms may also arise in the trachea ( Box 56.1 ).
Malignant Tracheal Neoplasms
Epithelial
Squamous cell carcinoma
Adenoid cystic carcinoma
Carcinoid
Mucoepidermoid carcinoma
Adenocarcinoma
Small cell carcinoma
Large cell carcinoma
Acinic cell carcinoma
Malignant salivary gland–type mixed tumors
Carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements
Mesenchymal
Fibrosarcoma
Rhabdomyosarcoma
Angiosarcoma
Kaposi sarcoma
Liposarcoma
Osteosarcoma
Leiomyosarcoma
Chondrosarcoma
Paraganglioma
Spindle cell sarcoma
Lymphoma
Malignant fibrous histiocytoma
Benign Tracheal Neoplasms
Epithelial
Squamous cell papilloma
Papillomatosis
Pleomorphic adenoma
Glandular papilloma
Adenomas of salivary gland type
Mucous gland adenoma
Monomorphic adenoma
Oncocytoma
Mesenchymal
Hamartoma
Neurofibroma
Chondroma
Fibroma
Hemangioma
Granular cell tumor
Schwannoma
Fibrous histiocytoma
Pseudosarcoma
Hemangioendothelioma
Leiomyoma
Chondroblastoma
Lipoma
Glomus tumor
Prevalence and Epidemiology
Primary tracheal neoplasms are rare. It has been estimated that a primary tracheal tumor is roughly 180 times less common than a primary lung cancer. In a retrospective chart review of all cases of primary tracheal malignancy seen at the MD Anderson Cancer Center between 1945 and 2005, the authors identified 74 patients with primary tracheal cancers. Among these, 34 (46%) were squamous cell carcinomas, 19 (26%) were adenoid cystic carcinomas, and 21 (28%) were of other histologic types. Squamous cell carcinoma and squamous cell papilloma have a male predominance and are associated with cigarette smoking, whereas adenoid cystic carcinoma has no sex predilection and is not related to smoking.
Clinical Presentation
Primary tracheal neoplasms are often clinically silent until the tracheal lumen is narrowed by approximately 75%. At the time of clinical presentation, symptoms include dyspnea, cough, hemoptysis, wheezing, and stridor. Of interest, up to one-third of adult patients with primary tracheal neoplasms are initially misdiagnosed with “adult-onset” asthma. Thus this diagnosis should always raise the suspicion for a tracheal neoplasm.
The average age at presentation of squamous cell carcinoma is between 50 and 60 years. In contrast, patients with adenoid cystic carcinoma typically present approximately 1 decade earlier. However, adenoid cystic carcinoma may occur over a broad spectrum of ages, ranging from the third to the ninth decade. The average age at presentation for squamous cell papilloma is approximately 50 years, whereas diffuse papillomatosis most commonly presents in childhood.
Pathophysiology
Anatomy
Tracheal neoplasms are usually characterized by an endoluminal tracheal mass, which may have smooth, irregular, or lobulated margins. Although some overlap exists between benign and malignant lesions, benign lesions are typically less than 2 cm in diameter, with well-defined smooth borders and without evidence of contiguous tracheal thickening or mediastinal invasion. In contrast, malignant lesions usually vary in size between 2 and 4 cm in diameter, with a flat or polypoid shape and irregular or lobulated borders. Contiguous tracheal wall thickening and mediastinal invasion are frequently observed.
Manifestations of the Disease
Radiography
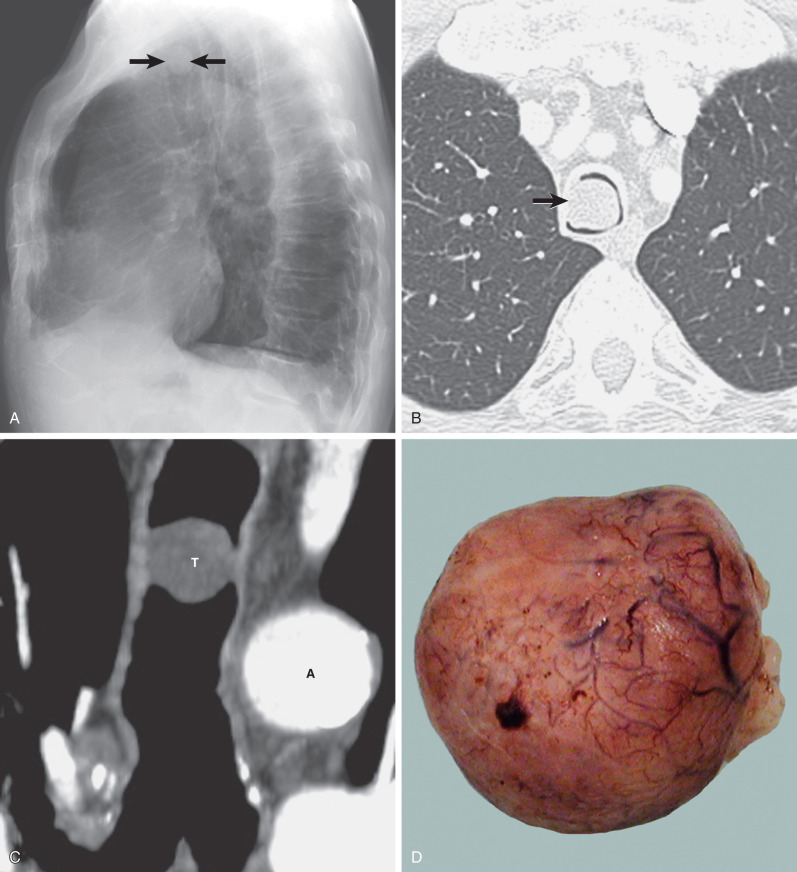
On careful scrutiny, an intraluminal tracheal mass is often visible on chest radiographs of patients with tracheal neoplasms ( Fig. 56.1 ). However, these lesions are frequently initially overlooked. Extraluminal involvement is not usually detectable by radiographs unless it is sufficiently extensive to distort the normal mediastinal contours.
Computed Tomography
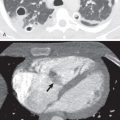
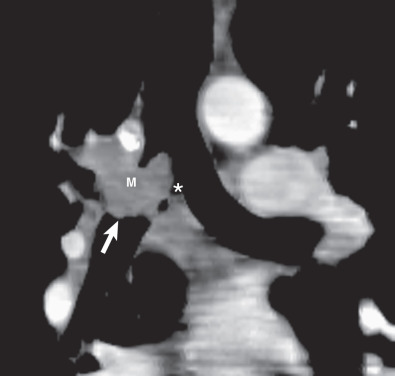
Multidetector CT has a high sensitivity (97%) for detection of tracheal neoplasms and is typically used for characterization of morphology and the extent of involvement of the trachea and adjacent structures. On CT a tracheal mass typically appears as a polypoid or sessile intraluminal mass of soft tissue attenuation ( Fig. 56.2 ; see also Fig. 56.1 ). Necrosis and ulceration may be observed, especially in squamous cell carcinomas. CT scans frequently suggest whether a lesion is malignant or benign (see Figs. 56.1 and 56.2 ). Although CT usually cannot distinguish between neoplastic cell types, detection of fat within a lesion on CT is nearly pathognomonic for a hamartoma or lipoma ( Fig. 56.3 ), and identification of calcification within a lesion is highly suggestive of a chondroid tumor (chondroma, chondrosarcoma).
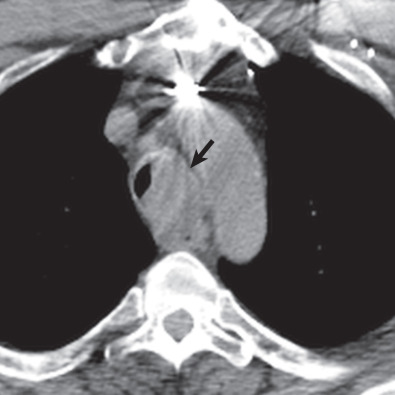
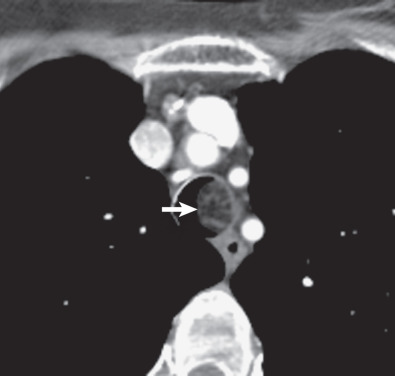
CT also provides an assessment of submucosal spread and local extratracheal invasion (see Fig. 56.2 ). Regional lymph node metastases and complications such as tracheoesophageal fistula may also be detected by CT. However, CT does not reliably detect microscopic mediastinal invasion or neural invasion.
Eccentric or circumferential tracheal wall thickening is less common than an intraluminal mass as a manifestation of malignant tracheal neoplasms. This feature is not associated with benign tracheal neoplasms. CT readily detects the presence of circumferential tracheal wall thickening and luminal narrowing. It readily distinguishes an intrinsic tracheal abnormality from extrinsic compression. Multiplanar and 3D reconstructions complement axial images by enhancing the accuracy of assessment of craniocaudad extent of disease and extratracheal extension ( Fig. 56.4 ).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is useful for tissue characterization and assessment of mediastinal invasion. Although most tracheal neoplasms have intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted images, MRI may show characteristic signal patterns in cases of leiomyomas, fibromas, hamartomas, and lipomas.
Imaging Algorithms
Chest radiographs are typically the first-line imaging test for a patient presenting with central airway symptoms. However, a high level of suspicion is required, as lesions are often overlooked at routine imaging.
CT is the imaging modality of choice for the detection and staging of tracheal neoplasms. MRI plays a secondary role complementary to CT in selected cases in which additional information is deemed necessary with regard to tissue characterization or when CT is indeterminate for mediastinal invasion. MRI is more sensitive than CT for detecting mediastinal invasion or submucosal growth of tumor.
Fluorodeoxyglucose–positron emission tomography (FDG-PET)–CT can be helpful in the diagnosis and staging of tracheal malignancies. Tracheal malignancies are almost universally FDG avid, and PET is highly sensitive for the detection of nodal metastases. PET-CT is more sensitive for the detection of tracheal tumors than CT alone and may be helpful in characterizing the full extent of tumors with submucosal spread, such as adenoid cystic carcinoma. In addition, PET-CT can be used to detect tumor recurrence after treatment.
Differential Diagnosis
From Clinical Data
Primary tracheal neoplasms are frequently misdiagnosed clinically as adult-onset asthma. Thus this diagnosis should prompt careful assessment of the trachea on chest radiographs. The clinical symptoms of central airway obstruction also overlap with other tracheal disorders, including tracheal stenosis and tracheomalacia. Thus it is not possible to establish a diagnosis of a tracheal tumor on clinical grounds alone.
From Supportive Diagnostic Techniques
When a discrete tracheal mass is identified on imaging studies, the diagnosis of a primary tracheal neoplasm can usually be made with a high degree of confidence. Cross-sectional imaging, including CT and MRI, readily differentiate a primary tracheal neoplasm from invasion by an extratracheal malignant neoplasm, such as thyroid carcinoma. In addition, CT and MRI can often distinguish benign and malignant lesions and may rarely provide a specific diagnosis. Although a discrete tracheal mass usually represents a primary tracheal neoplasm, the differential diagnosis also includes metastatic disease from an extrathoracic primary neoplasm, including breast cancer, colorectal carcinoma, renal carcinoma, melanoma, and lung cancer. A variety of nonneoplastic lesions, including granulation polyps, amyloidosis, and retained secretions, may also mimic a tracheal neoplasm on imaging studies. Secretions can sometimes be differentiated from a fixed tracheal lesion by identification of small foci of air within secretions; strands of additional secretions within the airway may also be identified. Alternatively, a repeat CT in a prone position after coughing can document a change in position or clearing of secretions.
In contrast, when a tracheal neoplasm manifests as eccentric or circumferential tracheal wall thickening rather than as a discrete mass, the differential diagnosis includes tracheal stenosis from a variety of nonmalignant entities, including iatrogenic (postintubation stenosis), infectious (tuberculosis), and inflammatory causes (e.g., granulomatosis with polyangiitis, relapsing polychondritis).
In general, the presence of marked irregularity of tracheal wall thickening and the presence of extratracheal extension favor a primary tracheal neoplasm, but biopsy is usually necessary to establish the diagnosis.
- •
Tracheal neoplasms are usually malignant and most commonly manifest as an intraluminal tracheal mass and less commonly as eccentric or circumferential tracheal wall thickening.
- •
The most common cell types are squamous cell carcinoma and adenoid cystic carcinoma.
- •
Features suggestive of malignancy include size larger than 2 cm, irregular margins, contiguous tracheal wall thickening, and extratracheal extension.
- •
Tracheal neoplasms are often overlooked on chest radiographs but can usually be identified retrospectively on careful inspection of the trachea.
- •
CT is the imaging modality of choice; multiplanar and 3D reconstructions enhance assessment of craniocaudal extent of disease and extratracheal involvement.
Types of Tracheal Neoplasms
Squamous Cell Carcinoma
Squamous cell carcinomas are often large (4 cm) at the time of initial diagnosis, and their endoluminal component may be either exophytic or ulcerative. These lesions are often sessile and frequently result in asymmetric narrowing of the tracheal lumen. Regional lymph node metastases and local mediastinal invasion are relatively common. Extension from the trachea into the main bronchus and development of a tracheoesophageal fistula are observed in 25% and 15% of cases, respectively.
Adenoid Cystic Carcinoma
Adenoid cystic carcinoma is the most common variety of tracheobronchial gland neoplasm, accounting for about 75% to 80% of cases. It usually arises from the trachea or the main bronchi and only occasionally from more distal bronchi or lung periphery. The mean age at diagnosis is 45 to 50 years. Cigarette smoking does not seem to be an etiologic factor. The most common clinical manifestations are cough, hoarseness, hemoptysis, dyspnea, wheeze, and recurrent pneumonitis. The symptoms of dyspnea and wheeze frequently lead to a misdiagnosis of asthma.
Adenoid cystic carcinomas grossly appear as polypoid or broad-based and infiltrative intraluminal tracheal masses. At diagnosis, the lesions are usually less than 2 cm, and their surface may be either smooth or ulcerated. These neoplasms are nonencapsulated and characteristically spread along perineural surfaces or perineural lymphatics. At the time of diagnosis, the lesion has often already infiltrated long segments of the tracheal submucosa. The extent of microscopic invasion is typically greater than that estimated by imaging assessment and direct visualization at surgery.
CT is superior to radiography in identifying the tumors and is particularly helpful in assessing the presence of extraluminal extent and mediastinal invasion. The most common finding on CT consists of a lobulated, polypoid endoluminal mass with associated focal thickening of the airway wall ( Fig. 56.5 ). Less commonly, adenoid cystic carcinoma may be associated with extensive thickening of the tracheal or bronchial wall ( Fig. 56.6 ). Adenoid cystic carcinoma has a tendency to infiltrate beneath the mucosa; its longitudinal extent may be underestimated on CT. Optimal assessment requires thin sections (preferably 1 mm or less) and multiplanar or 3D reformations.
- •
Adenoid cystic carcinoma is the most common tracheobronchial gland neoplasm and the second most common primary malignant tumor of the trachea (after squamous cell carcinoma).
- •
Most tumors arise in the trachea or main bronchi.
- •
Imaging findings include the following:
- •
Polypoid endoluminal mass
- •
Circumferential tracheal wall thickening
- •
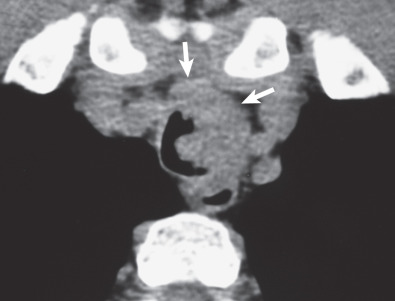
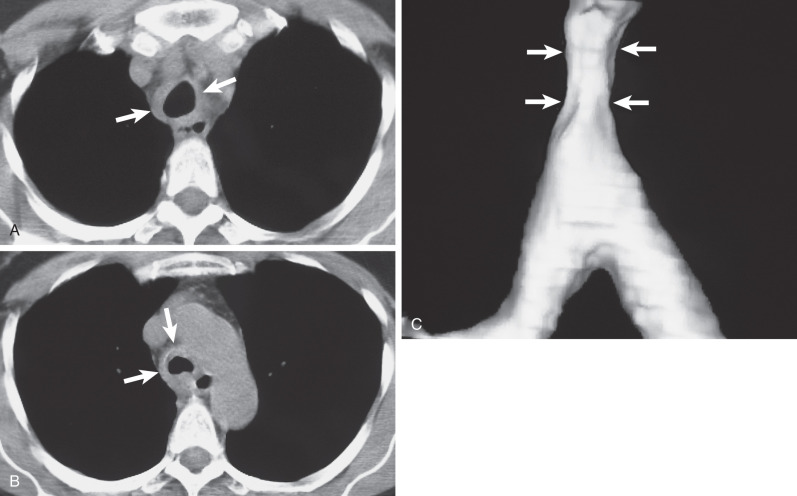
Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma (MEC) is the second most common form of tracheobronchial gland neoplasm but accounts for less than 0.2% of pulmonary carcinomas. Patients vary in age from 3 months to 78 years, but almost half are younger than 30 years. Symptoms are related predominantly to growth in the airway wall and lumen and include cough, hemoptysis, recurrent pneumonia, and dyspnea.
MECs are composed of cells that show glandular (typically mucus production) and “epidermoid” features and are classified histopathologically into low- and high-grade neoplasms. They usually are located in segmental bronchi and, less commonly, arise within the lobar or main bronchi or trachea. Most grow within the airway lumen and produce a polypoid mass with an intact or occasionally ulcerated surface epithelium; peripheral extension within the bronchial lumen may occur. Low-grade tumors often are confined to the bronchial wall; by contrast, the high-grade form commonly extends into the peribronchial interstitium or adjacent lung parenchyma with propensity for lymph node metastases.
Radiographic findings are related to tumor location and size and may consist of a solitary nodule or mass, lobar or segmental consolidation or atelectasis, or a central mass with associated obstructive pneumonitis or atelectasis ( Fig. 56.7 ).
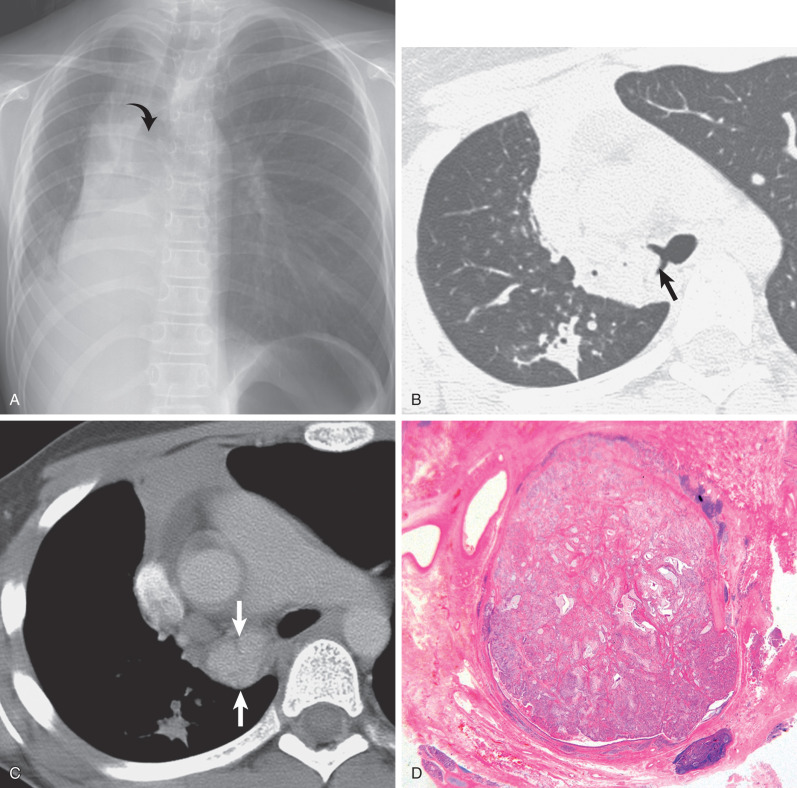
The CT manifestations of MECs usually consist of a smoothly oval or lobulated soft tissue nodule or mass measuring at least 1 cm in diameter. Punctate calcification within the tumor is seen on CT scan in 25% to 50% of cases. High-grade MECs typically show high FDG uptake, whereas low-grade tumors show low uptake that may be similar to a mediastinal blood pool. The tumors are homogeneous and show slight enhancement after the administration of contrast medium ( Fig. 56.8 ). The direction of the longest diameter of the oval or lobulated tumor in the lobar or segmental bronchus is typically parallel to that of the branching pattern in the corresponding airways containing the tumor. Associated CT findings include distal bronchial dilation with mucoid impaction, postobstructive pneumonia, atelectasis, and air-trapping.
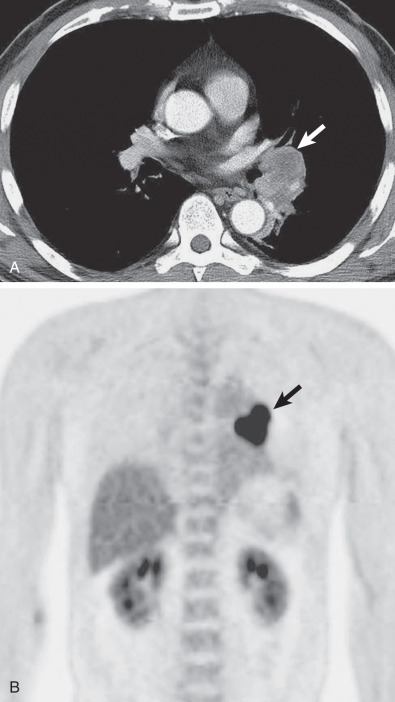
Occasionally, the tumors may involve the trachea rather than the bronchi and appear as a polypoid intraluminal nodule on radiograph and CT scan ( Fig. 56.9 ). CT is superior to radiographs in the assessment of intraluminal tumor and the extent of involvement of the bronchial or tracheal wall and mediastinum. Metastatic disease, including hilar or mediastinal lymphadenopathy, pleural nodularity, osseous lesions, and liver lesions, is uncommon except in high-grade MECs.
- •
Mucoepidermoid carcinoma (MEC) accounts for less than 0.2% of pulmonary carcinomas.
- •
MEC most commonly involves segmental bronchi.
- •
Imaging findings include the following:
- •
1- to 4-cm nodule or mass
- •
Distal obstructive pneumonitis, atelectasis, or air-trapping
- •
Close association with bronchus usually evident on CT
- •
Tracheal Papilloma
A solitary papilloma typically appears as a small, sessile or pedunculated growth arising from the tracheal wall. Larger lesions may demonstrate a cauliflower-like appearance. A range of squamous dysplasia, as well as in situ or invasive carcinoma, may also be identified within squamous cell papillomas.
Synopsis of Treatment Options
Medical
Medical therapy does not play a primary role in the treatment of tracheal tumors. Antibiotic therapy may be administered to treat postobstructive pneumonia or bronchitis.
Surgical
Surgery is the treatment of choice, with resection of the involved portion of the trachea and reconstruction of the remaining uninvolved portions of the trachea. Accurate preoperative identification of the precise level and length of resection is critically important.
Surgical treatment is usually curative for benign tracheal neoplasms. Complete excision of a solitary papilloma is recommended to exclude an invasive malignant neoplasm and to prevent recurrence.
Treatment of malignant tracheal neoplasms is usually by segmental resection and reconstruction performed in a single stage and adjuvant preoperative and postoperative irradiation. For patients in whom squamous cell or adenoid cystic carcinoma is unresectable, palliative methods that yield good success rates are available, including laser photoresection, external beam irradiation, and brachytherapy.
MECs have a 5-year survival after surgery of approximately 80% in patients with low-grade malignancy at histology and 30% in patients with carcinomas with high-grade malignancy. It is recommended that MECs be treated by radical surgery with lymph node sampling and dissection.
Tracheal Stenosis
Etiology
Tracheal stenosis refers to narrowing of the tracheal lumen. A variety of iatrogenic, inflammatory, infectious, and neoplastic processes may result in focal or diffuse tracheal narrowing. This section focuses on postintubation tracheal stenosis, which is by far the most common cause of acquired tracheal stenosis.
Postintubation stenosis occurs secondary to injury of the trachea from the high pressure of an endotracheal tube balloon against the wall of the trachea. This initially results in mucosal necrosis, followed by scarring and stenosis.
Prevalence and Epidemiology
The true prevalence of postintubation tracheal stenosis is unknown. Although it was initially reported in up to 20% of cases after endotracheal intubation, its prevalence has decreased substantially to an estimated 1% with the introduction of low-pressure cuff endotracheal tubes. The prevalence of tracheal stenosis has been estimated at approximately 30% after long-standing tracheostomy tube placement.
Risk factors include difficult or prolonged intubation, infection, mechanical irritation, steroid administration, and use of positive-pressure ventilation.
Clinical Presentation
Affected patients typically present with dyspnea on exertion, stridor, and wheezing. Symptoms of upper airway obstruction are often delayed several weeks after extubation. Patients with mild stenoses may initially be asymptomatic. However, such patients may eventually develop symptoms when tracheal luminal narrowing is worsened by airway edema and secretions from a coexistent respiratory infection.
Pathophysiology
Anatomy
Postintubation stenosis is characterized by eccentric or concentric tracheal wall thickening and associated luminal narrowing. The craniocaudal length usually ranges from 1.5 to 2.5 cm. In patients who have undergone tracheostomy tube placement, the stenosis occurs most commonly at the stoma site and less commonly at the site where the tip of the tube has impinged on the tracheal mucosa. In patients who have undergone endotracheal intubation, stenosis occurs most commonly in the subglottic region at the level of the endotracheal tube balloon.
Pathology
Acutely, ischemic necrosis is due to compromise of the blood supply to the tracheal mucosa. This is followed by a superficial tracheitis with shallow ulcerations. The exposed cartilaginous rings subsequently soften and become fragmented. This is followed by fibrosis and granulation tissue formation, resulting in concentric or eccentric wall thickening with associated luminal narrowing.
Lung Function
Flow-volume loops may show characteristic changes of airway obstruction. Such changes typically precede abnormalities in spirometric volumes.
Manifestations of the Disease
Focal Tracheal Narrowing
General
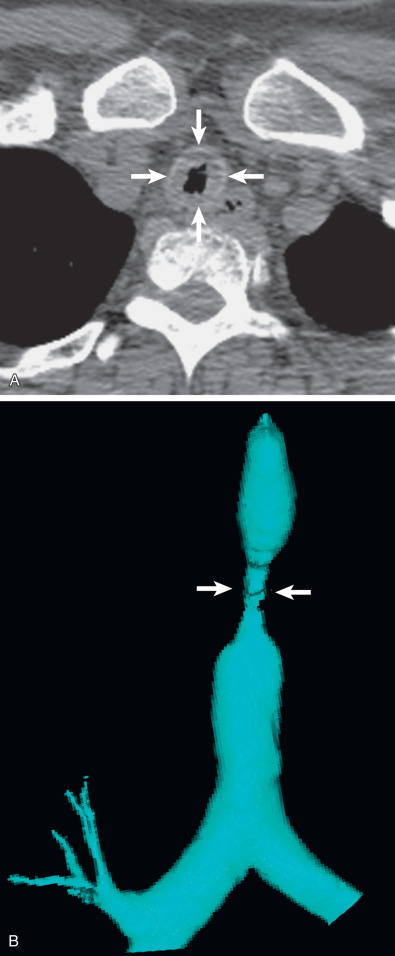
The most common imaging finding in postintubation stenosis is a focal area of tracheal luminal narrowing measuring approximately 2 cm in craniocaudal length. The focal nature and circumferential narrowing may produce a characteristic hourglass configuration.
Less commonly, one may observe a thin membrane projecting into the tracheal lumen or a long segment of eccentric soft tissue thickening.
Radiography
Postintubation stenoses are often overlooked on conventional radiographs, in part because of a proximal location. On careful scrutiny of radiographs, however, airway narrowing is usually visible in cases of significant stenosis.
Computed Tomography
CT is the imaging modality of choice to detect and characterize tracheal stenosis. On axial images, CT demonstrates eccentric or concentric soft tissue thickening with associated luminal narrowing ( Fig. 56.10 ). Multiplanar reconstruction and 3D external rendering of images help detect focal stenoses and aid in assessment of craniocaudal length, which is often underestimated on axial images ( Fig. 56.11 , see Fig. 56.10 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree