Breast Anatomy and Basic Histology, Physiology, and Pathology
As with all organ systems, an understanding of the anatomy, histology, and pathology of the breast leads to an enhanced ability to interpret imaging studies. Breast imaging primarily involves the assessment of the morphology of macroscopically visible breast structures. A basic understanding of the anatomy and histology of the breast, and of the complex underlying microscopic structures in which changes take place, is important for an understanding of the pathologic processes that occur. All are helpful for image interpretation.
Radiologists are all pattern readers. However, rather than just searching for patterns, the interpreter should try to understand the underlying processes that produce the morphologic changes that are visible on the various imaging studies. Although different processes may produce similar findings, in many cases the imaging morphology reflects histologic and pathologic changes. The anatomy of the breast and the organization and distribution of the histologic elements shape the imaging findings. Ideally the interpreter should be able to explain, using specific criteria, why a finding is judged benign or potentially malignant by being able to explain the specific characteristics of the finding that led to the particular assessment.
Anatomy Overview
The breast is a modified skin gland that develops from the mammary ridge in the embryo. It generally lies on the chest wall between the clavicle and the sixth to eighth ribs. Breast tissue can be found as far medially as the sternum and laterally to the midaxillary line. Breast tissue is frequently found high in the axilla, occasionally reaching to its apex.
The breast lies on top of and lateral to the pectoralis major muscle, whose muscle fibers course obliquely from the ribs to the humerus. Since breast tissue frequently wraps around the lateral margin of the pectoralis major muscle (Fig. 2A-1), imaging the breast is best accomplished using the mediolateral oblique projection. The best way to image the most tissue is by positioning the breast such that the plane of compression is parallel to the oblique fibers of the free margin of the muscle. This permits maximum traction on the breast so that it can be fully positioned over the detector and comfortably compressed. It also permits evaluation of the portion of the breast that lies lateral to the muscle and extends up into the axilla.
Recent work at the MGH by Jennifer Rusby, MD, suggests that there are usually more than 20 lobes or segments that are defined by the major lactiferous ducts that open on the nipple. A lobe (segment) can be thought of as a tree whose trunk, branches, and leaves are hollow. These arborizing networks conduct milk from the lobules (the true glands of the breast) to the nipple. The lobule is actually the most proximal of the structures if direction is the flow of breast secretions (milk), but it is called the terminal portion of the duct system. The lobule consists of multiple blunt-ending ductules in a cluster like the fingers of a glove. These fingers form the glandular acini of the lobule. They are surrounded by specialized connective tissue that differs histologically
from the stromal connective tissue found in the rest of the breast. The acini and specialized connective tissue together form the lobule. A terminal duct and its lobule are collectively called the terminal duct lobular unit (TDLU) (Fig. 2A-2). TDLUs can be found as immediate branches of the major ducts and are not always at the periphery of the ductal networks. Breast cancer is thought to originate in the terminal duct in the lobule. One theory is that this is the location of stem cells and that these undifferentiated cells are the most likely to develop malignant transformations.
from the stromal connective tissue found in the rest of the breast. The acini and specialized connective tissue together form the lobule. A terminal duct and its lobule are collectively called the terminal duct lobular unit (TDLU) (Fig. 2A-2). TDLUs can be found as immediate branches of the major ducts and are not always at the periphery of the ductal networks. Breast cancer is thought to originate in the terminal duct in the lobule. One theory is that this is the location of stem cells and that these undifferentiated cells are the most likely to develop malignant transformations.
The breast is held together by varying amounts of connective tissue that form varying volumes of sheets of tissue known as Cooper’s ligaments. Fat is interspersed throughout the breast and surrounds it as subcutaneous and retromammary adipose deposits.
Breast Development
Little detail is available on the actual development of the human breast. Much of the information that we have is based on mouse models, which differ significantly from the human breast. The breasts have the same ectodermal origin as skin glands. They develop from the mammary ridges, which begin as ventral “streaks” found in the fifth week of gestation. The mammary ridges extend longitudinally from the base of the forelimb bud (the primitive axilla) along the ventral surface of the embryo (the chest and abdomen to be) to an area medial to the base of the hindlimb bud (the primitive inguinal region). If development proceeds normally, the middle portion of the upper third of the mammary ridge persists to form the breast bud on the chest wall and eventually the tail of Spence, extending into the axilla, while the remainder of the structure disappears. Failure of portions of the ridge to involute may result in accessory breast tissue anywhere along the “milk line,” extending from the axilla to the inguinal region (1,2).
The axilla is the most common area in which accessory breast tissue can be found. Accessory breast tissue may be in continuity with the main breast tissue (Fig. 2A-3A), it may appear as a separate discontinuous structure (Fig. 2A-3B), or it may actually form a complete breast (Fig. 2A-3C). Accessory nipples are occasionally present (see Fig. 2A-3D). These are most common in the anterior superior abdominal wall just caudal to the usual breast location and may be mistaken for nevi.
Although very rare, breast cancer can occur anywhere there is a rest of breast tissue (3,4). We have seen a case where breast cancer developed in a rest of tissue in the upper anterior abdominal wall. Since the most common location for accessory breast tissue is in the axilla, imaging should include as much axillary tissue as possible.
During the first trimester of intrauterine growth, the primitive epidermal bud in the embryo begins to produce
cords of epithelial cells that penetrate down into the dermis. Research indicates that there are factors that are produced in the underlying mammary mesenchyme that stimulate this growth (5). Interactions between the glandular tissues of the breast and its supporting stroma appear to continue throughout life. In the full-term fetus there is already a simple network of branching ducts, and although lobules (the glandular elements) do not appear until adolescence, secretion may occur under the stimulation of maternal hormones and the newborn may have a nipple discharge.
cords of epithelial cells that penetrate down into the dermis. Research indicates that there are factors that are produced in the underlying mammary mesenchyme that stimulate this growth (5). Interactions between the glandular tissues of the breast and its supporting stroma appear to continue throughout life. In the full-term fetus there is already a simple network of branching ducts, and although lobules (the glandular elements) do not appear until adolescence, secretion may occur under the stimulation of maternal hormones and the newborn may have a nipple discharge.
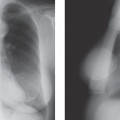
 Figure 2A-2 (A) This schematic representation of the basic anatomy of the breast can be compared to the lateral view (B) from a negative mode radiograph using the Xerox technique. |
Thelarche (breast development) usually precedes menarche, and under hormonal stimulation the breast buds enlarge, becoming palpable discs beneath the nipple. The ducts grow back into the soft tissues, which are also stimulated to increase, and lobular development (differentiation) begins. This growth may proceed asymmetrically because of the fluctuating hormonal environment and a variable sensitivity of the end-organ tissues to the stimulation (Fig. 2A-4). In my experience the only reason for one breast to be larger than the other is due to developmental asymmetry (Fig. 2A-5). On the rare occasion when one breast becomes larger due to cancer, the reason is obvious, since a breast full of tumor becomes extremely hard.
I have been unable to determine the true developmental sequence of the breast in the subcutaneous tissue. The breast develops in the superficial layer of fascia that lies just beneath the skin. It is not clear whether the superficial layer splits into a deep and superficial layer to form an incomplete envelope around the gland, or whether the elongating ducts invaginate the fascia, which then ends up enveloping the gland (Fig. 2A-6). One of our senior surgeons often spoke of a definable “capsule” that is often present around the glandular portion of the breast, suggesting the latter. With digital mammography and image processing I believe that this “capsule” can be seen on many mammograms (Fig. 2A-7).
Since the breast buds may develop asymmetrically, the detection of an asymmetric lump beneath the nipple before puberty should not be cause for concern (see Fig. 2A-4). Biopsy should rarely be considered, since the inadvertent removal of the breast bud will result in failure of breast development. Only the rarest forms of breast cancer occur in prepubertal and pubertal girls, and these are usually indolent. When they do occur, they are likely to grow eccentrically
from the nipple, unlike the breast bud, which is centered under the nipple. Asymmetry at this stage almost always resolves in ultimate fairly symmetrical breast development.
from the nipple, unlike the breast bud, which is centered under the nipple. Asymmetry at this stage almost always resolves in ultimate fairly symmetrical breast development.
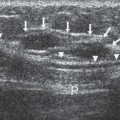
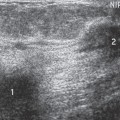
 Figure 2A-4 Ultrasound demonstrates asymmetric development of the normal breast buds in a 9-year-old girl. The breast buds are the hypoechoic, triangular areas that lie beneath the nipple. |
Asymmetric Breast Size
Although adult breasts frequently differ in size, marked asymmetry is relatively unusual (see Fig. 2A-5). Without skin or other changes to suggest inflammation or neoplasia, asymmetric breast size is virtually always a developmental phenomenon. On the rare occasion where the breast enlarges due to cancer, the problem is obvious: the breast becomes extremely hard and the skin is usually thickened. If one breast is considerably larger than the other but there is no thickening, then the enlargement is virtually always
developmental. I suspect that developmental asymmetry may become more obvious if a patient gains weight. Since the breast is a repository for fat, if the breasts are asymmetric to begin with, the expansion of the adipose tissue in the breast may result in what appears to be one breast that is enlarging.
developmental. I suspect that developmental asymmetry may become more obvious if a patient gains weight. Since the breast is a repository for fat, if the breasts are asymmetric to begin with, the expansion of the adipose tissue in the breast may result in what appears to be one breast that is enlarging.
 Figure 2A-5 This is a developmental anomaly where the left breast is much larger than the right, as seen on the craniocaudal projection. |
 Figure 2A-7 On digital mammography it is possible to see what appears to be a capsule around the parenchymal cone of the fibro-glandular tissue. |
Male breasts also contain primitive ducts. Probably as a result of the fluctuating hormonal environment in adolescent males, gynecomastia is fairly common during puberty (see Chapter 19, “The Male Breast”). This too may be asymmetric and almost always corrects itself without the need for intervention.
Breast Size and Risk of Breast Cancer
It is not clear that a larger breast means that there are a greater number of TDLUs. Since this is the location for the development of most breast cancers, one could speculate that women with more TDLUs might be at higher risk. However, I suspect that breast size and true “glandularity” are independent of one another. There are no good studies that have demonstrated any significant relationship between breast size and risk for cancer.
Terminal Differentiation
As the breast grows, the subcutaneous adipose and connective tissues increase in volume and ductal elements proliferate, elongating and extending deeper into the subcutaneous tissues. Over a variable period of time, terminal buds at the ends of the branching ducts differentiate into tufts of blunt-ending ductules that form the glandular acini of the TDLUs. The exact cells responsible for duct elongation and lobular differentiation have not been identified. The evidence suggests that there are “stem” cells at the terminal end of the duct that are responsible for this growth. Studies suggest that it may be the undifferentiated stem cell that is transformed and results in breast cancer (6). It is likely that the rapid cell proliferation and DNA replication that take place in this area account for the fact that most cancers appear to develop in the terminal duct as it enters, or along its course within, the lobule. Increased cellular proliferation increases the chance that DNA will not be copied properly and that mutations will occur.
Deng et al (7) found that there were similar genetic defects (loss of heterozygosity) in the cells of normal lobules that were adjacent to lobules that contained breast cancer cells, while cells in lobules further away did not have the same genetic changes. This suggests that there may be a common progenitor for both the benign and malignant cells of the affected duct network. If there is a stem cell responsible for the development of the ducts and lobules, then an alteration in its DNA early in life, prior to duct elongation and terminal lobular differentiation, could be distributed into every cell in the segment. This would place the cells throughout the segment at risk for further genetic change, increasing the likelihood that one of the cells might ultimately become a cancer. This would explain the findings of Deng et al. It might also be the explanation for the so-called field phenomenon, in which the cells in an area of the breast have a similar abnormality while other areas of the breast are normal. This is the likely explanation for diffuse adenosis, atypical hyperplasia, and lobular carcinoma in situ (LCIS).
It would be very unlikely that a carcinogen or mutagen could damage all of the cells in a segment while sparing cells in other segments of the mature breast. However, if a stem cell were altered in the immature breast, the damage would be distributed to all of the cells of the developing segment, and only the cells of that segment would be affected (Fig. 2A-8). This is the likely explanation for segmentally distributed abnormalities.
Our attention in preventing breast cancer is often directed toward older women. However, if there is a stem cell, its presence would suggest the importance of exposure
to carcinogens at an early age, prior to terminal differentiation. This is also supported by the fact that cancers with long doubling times (180 days) may take as long as 30 years to reach 2 cm in size (8), suggesting that malignant transformation for a woman who is diagnosed with cancer in her forties may have been initiated while she was in her teens or twenties. Data in mice suggest that the breast is susceptible to carcinogens during development. In mice, the period of end-bud differentiation is a time during which a carcinogen is more likely to initiate malignancy (9). A similar phenomenon may occur in humans. The data showing that radiation is a carcinogen in the breast show that the breast is more sensitive to radiation while it is developing, during adolescence and for women in their early twenties, while the mature (differentiated) breast of women in their early forties and older is not at increased risk from radiation. The maturation of the breast may take place over many years and may not be complete until the third decade of life, or it may proceed rapidly with an early first full-term pregnancy. It is likely that the terminal differentiation that takes place with a full-term pregnancy protects the breast from future carcinogens. Breast cancer prevention might include protecting the breast from carcinogens while it is developing.
to carcinogens at an early age, prior to terminal differentiation. This is also supported by the fact that cancers with long doubling times (180 days) may take as long as 30 years to reach 2 cm in size (8), suggesting that malignant transformation for a woman who is diagnosed with cancer in her forties may have been initiated while she was in her teens or twenties. Data in mice suggest that the breast is susceptible to carcinogens during development. In mice, the period of end-bud differentiation is a time during which a carcinogen is more likely to initiate malignancy (9). A similar phenomenon may occur in humans. The data showing that radiation is a carcinogen in the breast show that the breast is more sensitive to radiation while it is developing, during adolescence and for women in their early twenties, while the mature (differentiated) breast of women in their early forties and older is not at increased risk from radiation. The maturation of the breast may take place over many years and may not be complete until the third decade of life, or it may proceed rapidly with an early first full-term pregnancy. It is likely that the terminal differentiation that takes place with a full-term pregnancy protects the breast from future carcinogens. Breast cancer prevention might include protecting the breast from carcinogens while it is developing.
Additional lobular development may take place in preparation for lactation. After the cessation of lactation, many of the lobules involute. Since the breast must be complete for lactation, a full-term pregnancy likely causes rapid lobular differentiation. It is likely, however, that complete maturation does not occur until after a full-term pregnancy. This may explain why women who have a first full-term pregnancy by the age of 18 have a much lower risk of subsequently developing cancer than women who remain nulliparous or have their first child after age 30. An early full-term pregnancy offers some protection possibly by narrowing the period of time over which differentiation takes place and, consequently, the “window of opportunity” during which a carcinogen may be most effective in causing lasting damage. If, as appears to be the case, the mature breast (differentiated terminal buds into lobules) is less susceptible to carcinogens (e.g., radiation), this might account for the diminished risk of breast cancer among women who bear a child early in life.
Breast Anatomy
In most individuals, the bulk of the breast extends from the second to the seventh rib. Since breast tissues often curve around the lateral margin of the pectoralis major muscle (Fig. 2A-9), the orientation of the muscle is important for optimal mammographic positioning. The pectoralis major muscle spreads like a fan across the chest wall. Portions of the pectoralis major muscle attach to the clavicle, the lateral margin of the scapula, costal cartilage, and the aponeurosis of the external oblique muscles of the abdomen. All these fibers converge on and attach to the greater tubercle of the humerus. The free fibers predominantly run obliquely over the chest from the medial portion of the thorax toward the humerus (see Fig. 2A-1). The relationship of the breast to the pectoralis major muscle influences two-dimensional projectional imaging, such as mammography. Since the breast tissue is closely applied to the muscle, some of the lateral tissues can be imaged only through the muscle. As with any soft-tissue structure overlying muscle, it is easier to project the breast into the field of view by pulling it away from the chest wall and compressing it with the plane of compression paralleling the obliquely oriented muscle fibers of the pectoralis major muscle. To maximize the tissue imaged, the free portion of the muscle should be included in the field of view.
Although it has not been directly studied, there is likely a variable attachment of the pectoralis major muscle medially to the thoracic wall. I believe that in approximately 1% of women, a small tongue of muscle adjacent to the sternum is sufficiently free to be pulled into the field of view of the mammogram in the craniocaudal projection (Fig. 2A-10). This is not seen on the mediolateral oblique, since it is very difficult to pull this portion of the muscle into the machine in this projection. We have seen what we believe to be a variant of the muscle on a chest-wall lateral xeromammogram (Fig. 2A-11). This variable portion of the muscle (Fig. 2A-12) may be round, triangular, or flame-shaped and should not be mistaken for a mass.
When a flame-shaped structure that is almost completely
separate from the chest wall is seen medially (Fig. 2A-13A), it is likely the sternalis muscle that is being imaged (Fig. 2A-13B). The sternalis muscle is a thin muscle that runs parallel to the sternum and is found in 4% to 11% of individuals (10). It has been speculated that it was once an extension of the rectus abdominis, but it is not connected to this or any other muscle, and its origin and use are unknown. It appears to be of no functional value and can be unilateral or bilateral.
separate from the chest wall is seen medially (Fig. 2A-13A), it is likely the sternalis muscle that is being imaged (Fig. 2A-13B). The sternalis muscle is a thin muscle that runs parallel to the sternum and is found in 4% to 11% of individuals (10). It has been speculated that it was once an extension of the rectus abdominis, but it is not connected to this or any other muscle, and its origin and use are unknown. It appears to be of no functional value and can be unilateral or bilateral.
 Figure 2A-10 The density seen medially (arrow) on this craniocaudal projection is the pectoralis major muscle pulled into the field of view by the compression system. |
Although either pectoralis major or sternalis muscles can project into the craniocaudal mammographic image, care must be exercised to avoid dismissing a true medial mass as muscle, since breast cancers can occur in this portion of the breast. If there is any doubt, computed tomography (CT; Fig. 2A-13C) (11), magnetic resonance imaging (MRI; Fig. 2A-13D), or ultrasound can be used to confirm or exclude a mass. By placing a patient on her side in the CT scanner so that the breast on the side in question pulls toward the table (Fig. 2A-14), the sternalis muscle will be pulled by the breast, and a definitive diagnosis can be made if this is needed.
 Figure 2A-12 The tissue densities seen at the edge of the film on these craniocaudal projections, medially, are the pectoralis major muscles. |
The pectoralis minor muscle lies beneath the pectoralis major muscle, extending from the third, fourth, and fifth ribs to the coracoid process of the scapula. Occasionally it is seen on the mediolateral oblique projection as a second triangle of muscle high in the axilla above the pectoralis major muscle in the corner of the film (Fig. 2A-15). This is not the latissimus dorsi muscle, as some have speculated. In lymph node dissection of the axilla, lymph nodes beneath the pectoralis minor muscle are called level II lymph nodes.
The Vascular Supply of the Breast
Arterial blood flows from the axillary artery through the lateral thoracic artery to supply the upper outer quadrant of the breast. The central and medial portions of the breast are supplied from perforating branches of the internal mammary artery, which lies adjacent to and beneath the sternal border. Branches of the intercostal arteries provide blood to the lateral breast tissues, with some blood coming from the subscapular and thoracodorsal arteries.
Venous drainage is back through the axillary, internal mammary, and intercostal veins, providing three major routes for hematogenous metastasis.
Enervation
Nerves supplying the breast originate primarily from the anterior and lateral cutaneous branches of the thoracic intercostal nerves, with some enervation from the cervical plexus to the upper breast. The deep pain sensors in the breast appear to be variable. In many women, needle aspiration or needle localization with 20-gauge or smaller needles can be performed with a minimum of discomfort without the use of local anesthesia (12). In some women, however, it is extremely difficult to establish deep anesthesia. In our experience this is more likely if the breasts are extremely dense radiographically, which usually is due to fibrous connective tissue. It is merely speculation, but perhaps the nerves are more tightly tethered in these women and are not displaced from the needle path. Fibrous connective tissue may also prevent the anesthetic from reaching the nerves.
Breast pain is a fairly common symptom and is likely due to edema and swelling that occurs in many women with the normal hormone cycle. Mammography is rarely able to demonstrate the cause of breast pain. Although pain is almost always due to benign processes and breast cancer is usually painless, malignancy occasionally causes breast pain. Focal pain and, in our experience, a drawing sensation are the types of pain that can be associated, on rare occasions, with breast cancer. Some physicians believe that some types of breast pain are actually chest wall in origin and referred to the breast. The discomfort of costochondritis, for example, can be perceived as breast pain. This is apparent when pushing the breast against the chest wall causes pain but squeezing it side to side does not.
The Lymphatics of the Breast
The lymphatic drainage of the breast has diagnostic and therapeutic implications. Tumor can spread through the lymphatic vessels. The lymphatic system is also a route for access to the vascular system as lymph is eventually returned to the venous system through the thoracic duct and other anastomoses. Although breast cancer likely spreads primarily hematogenously, the presence of tumor in the lymphatics or in the lymph nodes indicates that the tumor has developed a metastatic potential. This increases the likelihood that tumor is elsewhere in the body.
Not all breast cancers have the ability to metastasize. Although it is not yet possible to determine which do have the ability to spread to other organs, lymph node involvement indicates that the tumor has achieved a metastatic capability and that the disease should be considered systemic and no longer confined to the breast. Some studies suggest that spread through the lymphatics may be the primary route for access to the vascular system, although pathologists
describe direct invasion of blood vessels within the breast. Lymph nodes containing tumor are usually either excised or treated with radiation to prevent recurrence, although treating the lymph nodes does not appear to influence overall survival. The lymphatics have gained increasing importance as sentinel lymph node biopsy (detection and removal of the first lymph nodes in the chain that drain the tissue containing cancer) has replaced full axillary dissection as the method of choice for assessing the status of the lymph nodes.
describe direct invasion of blood vessels within the breast. Lymph nodes containing tumor are usually either excised or treated with radiation to prevent recurrence, although treating the lymph nodes does not appear to influence overall survival. The lymphatics have gained increasing importance as sentinel lymph node biopsy (detection and removal of the first lymph nodes in the chain that drain the tissue containing cancer) has replaced full axillary dissection as the method of choice for assessing the status of the lymph nodes.
The lymphatic drainage of the breast has been extensively studied, yet significant uncertainty continues as to its distribution and how it actually functions. There are clearly significant variations between individuals. Some
investigators have suggested that the lymphatic drainage comes from the deeper tissues of the breast flowing toward the surface through lymphatic channels to the skin. These then drain into a subareolar plexus and then to the axilla. There is a small percentage of drainage to the upper abdomen and medially to the internal mammary lymphatic chain, but the primary lymphatic drainage from all parts of the breast (including the medial tissues) is to the axilla. That there is significant variation is clear in a small number of cases in which investigators injected an iodinated contrast into the tumor bed of women with cancer and into the tissues around the cancer and then imaged the lymphatics with multi-slice CT (13). Lymphatics are clearly visible coursing directly from these areas through the breast itself to the axilla; in one case there was drainage both toward the axilla and toward the internal mammary chain. These drainage routes are increasingly important as surgeons rely increasingly on sentinel lymph node identification and biopsy. The most common method for identifying the sentinel nodes at this time is to inject radioactive technetium sulfur colloid followed by the injection of a vital dye so that these are picked up by the lymphatics and flow toward the first lymph node in the drainage of the breast. Using a collimated Geiger counter, the radioactive lymph node is identified, and then the blue dye helps to make it visible during the limited dissection. The goal is to facilitate the passage of these materials to the sentinel or first lymph nodes in the lymphatic drainage of the breast.
investigators have suggested that the lymphatic drainage comes from the deeper tissues of the breast flowing toward the surface through lymphatic channels to the skin. These then drain into a subareolar plexus and then to the axilla. There is a small percentage of drainage to the upper abdomen and medially to the internal mammary lymphatic chain, but the primary lymphatic drainage from all parts of the breast (including the medial tissues) is to the axilla. That there is significant variation is clear in a small number of cases in which investigators injected an iodinated contrast into the tumor bed of women with cancer and into the tissues around the cancer and then imaged the lymphatics with multi-slice CT (13). Lymphatics are clearly visible coursing directly from these areas through the breast itself to the axilla; in one case there was drainage both toward the axilla and toward the internal mammary chain. These drainage routes are increasingly important as surgeons rely increasingly on sentinel lymph node identification and biopsy. The most common method for identifying the sentinel nodes at this time is to inject radioactive technetium sulfur colloid followed by the injection of a vital dye so that these are picked up by the lymphatics and flow toward the first lymph node in the drainage of the breast. Using a collimated Geiger counter, the radioactive lymph node is identified, and then the blue dye helps to make it visible during the limited dissection. The goal is to facilitate the passage of these materials to the sentinel or first lymph nodes in the lymphatic drainage of the breast.
There has been great debate as to the best site in which to inject these materials. There are proponents of injection around the tumor and those who favor injection into the subcutaneous tissue; others favor an intradermal injection, and still others believe a subareolar injection is the best. Each has its proponents, and it only depends on the published series as to which one adheres. I suspect that most injection sites work as long as they are in the correct breast! There is certainly a variation that is based on the individual, and there is no one injection site that appears to be the best for all situations.
Although the concept of staging breast cancer is changing, the presence of tumor in the lymphatics within the breast as well as in the axilla is of important prognostic significance. Treatment may be modified based on analysis of the lymphatics and axillary nodes. For staging purposes and prognostication, axillary lymph nodes are divided into three levels (Fig. 2A-16). Level I lymph nodes are lateral to the lateral margin of the pectoralis major muscle and extend down into the tail of the breast. Mammography can frequently demonstrate the lowest lymph nodes in this portion of the lymphatic chain. Level II lymph nodes are beneath the pectoralis minor muscle. When an axillary dissection is performed, the free margin of the pectoralis major muscle is retracted out of the way and preserved, and the pectoralis minor muscle is transected to remove these lymph nodes. This is also performed when a modified radical mastectomy is used to treat breast cancer. The level III lymph nodes are medial and superior to the pectoralis minor muscle, up to the clavicle. Data suggest that the higher the level of nodal involvement with cancer, the worse the prognosis.
Sentinel Node
Most of the lymphatic drainage of the breast usually converges on the chain of lymph nodes described above. There are often one or two lymph nodes in the axilla through which most of the lymph drainage from the breast passes (Fig. 2A-17). These have been called the sentinel nodes. Sentinel nodes were first demonstrated in patients with melanoma: technetium-99m sulfur colloid, injected at the periphery of the melanoma, concentrated in the first lymph nodes that drained the area of the tumor (14), making them identifiable using a gamma probe (15,16). Removal and examination of these nodes was predictive for the presence or absence of metastatic disease. By reducing the need for more complex nodal dissection, morbidity and expense were reduced.
Krag (17) and Giuliano (18) pioneered the evaluation of the sentinel nodes in the axilla in women with breast cancer. In Krag’s preliminary report, 0.4 mCi technetium-99m sulfur colloid mixed in 0.5 mL saline was injected into the tissue in a 180-degree arc around the tumor along its axillary perimeter, and a probe was held over the axilla. The sentinel node was found where there were at least 30 counts in 10 seconds. The node was removed and evaluated for the presence of metastatic disease and compared to the results of the full axillary dissection. In a study of 22
women, a sentinel node was identified in 18 women who had full axillary dissections (all three levels) following radionuclide injections 1 to 9 hours before surgery. Among the 18 women, 62 radioactive and 170 nonradioactive nodes were removed. In all seven of the women who had positive nodes, the sentinel node contained tumor. In three of the patients, only this node contained tumor.
women, a sentinel node was identified in 18 women who had full axillary dissections (all three levels) following radionuclide injections 1 to 9 hours before surgery. Among the 18 women, 62 radioactive and 170 nonradioactive nodes were removed. In all seven of the women who had positive nodes, the sentinel node contained tumor. In three of the patients, only this node contained tumor.
Although we originally injected around the tumor, injections in the periaveolar region appears to be just as effective and keeps tracer at the injection site away from axilla where it could hide signals from the nodes (18a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree