Dementia with Lewy bodies (DLB) is a relative newcomer to the field of late-life dementia. Although a diversity of imaging methodologies is now available for the study of dementia, these have been applied most often to Alzheimer’s disease (AD). Studies on DLB, although fewer, have yielded fascinating and important insights into the underlying pathophysiology of this condition and allowed clinical differentiation of DLB from other dementias. Imaging research on DLB has had significant ramifications in terms of raising the profile of DLB and helping define it as a distinctive and separate disease entity from AD.
History
Dementia with Lewy bodies (DLB) is a relative newcomer to the field of late-life dementia, and its late emergence as an independent disease entity can in part be explained by its history. Friedrich Lewy initially noted the presence of spherical neuronal inclusions in autopsy cases of paralysis agitans, which were later coined Lewy bodies (LBs). Later in 1961, a case report by Okazaki and colleagues described two curious cases of elderly men who presented with dementia but who died shortly after with severe extrapyramidal rigidity, and who at autopsy had widespread LBs in the cerebral cortices. Over the next 20 years, further cases of dementia with parkinsonism were reported by Japanese researchers. Nevertheless, LB dementia was considered a rare condition, with the frequent co-occurrence of Alzheimer’s pathology (see later discussion) evident in the autopsy cases. Several competing labels were used, including diffuse LB disease, dementia associated with cortical LBs, the LB variant of Alzheimer’s disease (AD), senile dementia of LB type, and LB dementia.
Real interest in DLB, however, emerged in the late 1980s with advances in neuropathologic techniques including ubiquitin staining, which made the visualization of LBs easier. Seminal work done in a series of careful autopsy studies identified that LBs were actually a common feature of late-life dementia, affecting between 15% and 20% of elderly demented cases, and thus establishing DLB as the second most common form of degenerative dementia in old age. More recently, specific α-synuclein immunohistochemical staining has shown α-synuclein to be the core element of LBs, therefore placing DLB at the heart of the pathologic family of α-synucleinopathies that includes diseases such as Parkinson disease (PD), the associated Parkinson disease dementia (PDD), multisystem atrophy, pure autonomic failure, and rapid eye movement (REM) sleep behavior disorder. Further advances have shown that small aggregates of α-synuclein, not necessarily associated with LBs, are widespread in the brains of people with DLB, and it has been speculated that these small deposits may directly impair synaptic function, upset neuronal homeostatic energy processing, and lead ultimately to neuronal cell death. Nevertheless, linking how the pathologic changes lead to cognitive dysfunction and the other deleterious symptoms in DLB remains unclear.
Clinical characterization
Concordant with advances in neuropathology, research into DLB has led to the clearer clinical characterization of DLB, with the development of operationalized consensus criteria initially published in 1996 and revised in 2005 ( Box 1 ). The criteria clinically define 3 core symptoms for DLB: prominent (vivid) and recurrent visual hallucinations, spontaneous extrapyramidal symptoms, and fluctuating cognitive impairment. Other important clinical features are REM sleep behavior disorder and neuroleptic (antipsychotic) sensitivity. The presence of at least one core symptom plus one of the other supportive features is sufficient to make a diagnosis of probable DLB in the absence of obvious other causes such as cerebrovascular disease. Of note is the significance of neuroimaging in the revised criteria (discussed more in relevant sections in this article). Deficits in striatal dopamine transporter function are strongly suggestive of a diagnosis of DLB; that is, the presence of this plus only one of the core symptoms would be sufficient to make a diagnosis of probable DLB. Supportive neuroimaging features in making a diagnosis of DLB include relative preservation of medial temporal lobe structures on neuroimaging, generalized reductions in perfusion/metabolic imaging with reduced occipital activity, and abnormal cardiac imaging with iodine 123 meta -iodobenzylguanidine ([ 123 I]MIBG).
- 1.
Central feature (essential for a diagnosis of possible or probable DLB)
Dementia defined as a progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function. Prominent or persistent memory impairment may not necessarily occur in the early stages but is usually evident with progression. Deficits on tests of attention, executive function, and visuospatial ability may be especially prominent.
- 2.
Core features (2 core features are sufficient for a diagnosis of probable DLB, 1 for possible DLB)
- a.
Fluctuating cognition with pronounced variations in attention and alertness
- b.
Recurrent visual hallucinations that are typically well formed and detailed
- c.
Spontaneous features of parkinsonism
- a.
- 3.
Suggestive features (if one or more of these is present in the presence of one or more core features, a diagnosis of probable DLB can be made)
In the absence of any core features, one or more suggestive features is sufficient for the diagnosis of possible DLB. Probable DLB should not be diagnosed on the basis of suggestive features alone.
- a.
REM sleep behavior disorder
- b.
Severe neuroleptic sensitivity
- c.
Low dopamine transporter uptake in basal ganglia demonstrated by single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging
- a.
- 4.
Supportive features (commonly present but not proved to have diagnostic specificity)
- a.
Repeated falls and syncope
- b.
Transient, unexplained loss of consciousness
- c.
Severe autonomic dysfunction, eg, orthostatic hypotension, urinary incontinence
- d.
Hallucinations in other modalities
- e.
Systematized delusions
- f.
Depression
- g.
Relative preservation of medial temporal lobe structures on structural imaging
- h.
Generalized low uptake on SPECT/PET perfusion scan with reduced occipital activity
- i.
Abnormal (low uptake) MIBG myocardial scintigraphy
- j.
Prominent slow-wave activity on electroencephalogram with temporal lobe sharp waves
- a.
- 5.
A diagnosis of DLB is less likely
- a.
In the presence of cerebrovascular disease evident as focal neurologic signs or on brain imaging
- b.
In the presence of any other physical illness or brain disorder sufficient to account in part or in total for the clinical picture
- c.
If parkinsonism only appears for the first time at a stage of severe dementia
- a.
- 6.
Temporal sequence of symptoms
- a.
DLB should be diagnosed when dementia occurs before or concurrently with parkinsonism (if present). In research studies, when a distinction needs to be made between DLB and PDD, the 1-year rule between the onset of dementia and parkinsonism should be applied.
- a.
Clinical characterization
Concordant with advances in neuropathology, research into DLB has led to the clearer clinical characterization of DLB, with the development of operationalized consensus criteria initially published in 1996 and revised in 2005 ( Box 1 ). The criteria clinically define 3 core symptoms for DLB: prominent (vivid) and recurrent visual hallucinations, spontaneous extrapyramidal symptoms, and fluctuating cognitive impairment. Other important clinical features are REM sleep behavior disorder and neuroleptic (antipsychotic) sensitivity. The presence of at least one core symptom plus one of the other supportive features is sufficient to make a diagnosis of probable DLB in the absence of obvious other causes such as cerebrovascular disease. Of note is the significance of neuroimaging in the revised criteria (discussed more in relevant sections in this article). Deficits in striatal dopamine transporter function are strongly suggestive of a diagnosis of DLB; that is, the presence of this plus only one of the core symptoms would be sufficient to make a diagnosis of probable DLB. Supportive neuroimaging features in making a diagnosis of DLB include relative preservation of medial temporal lobe structures on neuroimaging, generalized reductions in perfusion/metabolic imaging with reduced occipital activity, and abnormal cardiac imaging with iodine 123 meta -iodobenzylguanidine ([ 123 I]MIBG).
- 1.
Central feature (essential for a diagnosis of possible or probable DLB)
Dementia defined as a progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function. Prominent or persistent memory impairment may not necessarily occur in the early stages but is usually evident with progression. Deficits on tests of attention, executive function, and visuospatial ability may be especially prominent.
- 2.
Core features (2 core features are sufficient for a diagnosis of probable DLB, 1 for possible DLB)
- a.
Fluctuating cognition with pronounced variations in attention and alertness
- b.
Recurrent visual hallucinations that are typically well formed and detailed
- c.
Spontaneous features of parkinsonism
- a.
- 3.
Suggestive features (if one or more of these is present in the presence of one or more core features, a diagnosis of probable DLB can be made)
In the absence of any core features, one or more suggestive features is sufficient for the diagnosis of possible DLB. Probable DLB should not be diagnosed on the basis of suggestive features alone.
- a.
REM sleep behavior disorder
- b.
Severe neuroleptic sensitivity
- c.
Low dopamine transporter uptake in basal ganglia demonstrated by single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging
- a.
- 4.
Supportive features (commonly present but not proved to have diagnostic specificity)
- a.
Repeated falls and syncope
- b.
Transient, unexplained loss of consciousness
- c.
Severe autonomic dysfunction, eg, orthostatic hypotension, urinary incontinence
- d.
Hallucinations in other modalities
- e.
Systematized delusions
- f.
Depression
- g.
Relative preservation of medial temporal lobe structures on structural imaging
- h.
Generalized low uptake on SPECT/PET perfusion scan with reduced occipital activity
- i.
Abnormal (low uptake) MIBG myocardial scintigraphy
- j.
Prominent slow-wave activity on electroencephalogram with temporal lobe sharp waves
- a.
- 5.
A diagnosis of DLB is less likely
- a.
In the presence of cerebrovascular disease evident as focal neurologic signs or on brain imaging
- b.
In the presence of any other physical illness or brain disorder sufficient to account in part or in total for the clinical picture
- c.
If parkinsonism only appears for the first time at a stage of severe dementia
- a.
- 6.
Temporal sequence of symptoms
- a.
DLB should be diagnosed when dementia occurs before or concurrently with parkinsonism (if present). In research studies, when a distinction needs to be made between DLB and PDD, the 1-year rule between the onset of dementia and parkinsonism should be applied.
- a.
Diagnosis of dementia with LBs versus PD with dementia
An area that has been subject to controversy is the separation of DLB from PDD. Consensus criteria for DLB and PDD recommend that for a diagnosis of PDD the extrapyramidal motor features need to be present for at least 12 months or more before the onset of the dementia, but if the dementia precedes the motor symptoms or has occurred within 12 months of the motor features, the diagnosis should be DLB. While subtleties exist between DLB and PDD, both conditions share cognitive, neuropsychiatric, neurochemical, pathologic, and imaging similarities, and this has led to the argument that both PDD and DLB should be considered the same disorder, or at the least two different points on an LB disease spectrum. The arbitrary one-year rule continues to allow distinction for research purposes and understanding the interrelationship between the two disease entities, but clinically, particularly where there is uncertainty concerning the onset of the motor symptoms relative to the cognitive symptoms, unitary approaches should be considered, and the catch-all term of LB dementias that includes both DLB and PDD can be useful in this regard.
Diagnosis of DLB versus AD
Recognition of DLB is important diagnostically because of the potentially dangerous adverse reaction to neuroleptics, the misuse of antiparkinsonian drugs, and the positive response to cholinesterase inhibitors. It has also been recognized that levels of neuropsychiatric symptomatology, functional disability, impaired quality of life, increased hospitalization, and annual care costs in DLB are significantly greater than those associated with AD, and thus early and accurate diagnosis is likely to be an essential component of effective treatment and management.
However, despite the development of clinical criteria, it is still apparent that DLB is underdiagnosed. Whereas 15% to 20% of dementia cases demonstrate neuropathologic features of DLB, epidemiologic surveys have tended to show rates of DLB to be much lower, at between 1% and 5%. This discrepancy has in part been driven by the lack of awareness of DLB as an independent clinical entity, difficulties in making the diagnosis clinically, and the interaction between coexistent AD and LB pathologies. Neuropathologically, up to 90% of DLB cases have high senile plaque counts, a level comparable with those found in pure AD, although only a minority of DLB cases show typical AD-associated neocortical neurofibrillary tangles, which are now thought to be integral to the pathophysiology of AD. Amyloid-β in the neocortex has also been associated with more extensive α-synuclein lesions, and thus it has been speculated that amyloid-β may have a role in the genesis of α-synuclein deposition in DLB.
Recent clinicopathologic data have suggested that the expression of a DLB clinical phenotype is related to the severity of LB pathology and inversely related to the severity of Alzheimer-type pathology ( Table 1 ). This finding has significant ramifications in making it difficult to diagnose DLB on clinical grounds alone in all cases.
| Alzheimer-Type Pathology | ||||
|---|---|---|---|---|
| NIA-Reagan Low | NIA-Reagan Intermediate | NIA-Reagan High | ||
| (Braak Stage 0–II) | (Braak Stage III–IV) | (Braak Stage V–VI) | ||
| LB type pathology | Brainstem predominant | Low | Low | Low |
| Limbic (transitional) | High | Intermediate | Low | |
| Diffuse neocortical | High | High | Intermediate | |
Neuroimaging in DLB
One of the main areas for advancing research and improving the diagnosis of DLB has been the use of imaging, and the following sections explore the use of several imaging modalities that have been applied in DLB. These have focused mainly on imaging of brain structures and function although, given the increasing recognition that DLB is a multisystem disorder, a discussion is included on the use of cardiac imaging with [ 123 I]MIBG to differentially diagnose DLB.
Structural magnetic resonance imaging
Volumetric changes in dementia, whether global or region specific, can be elucidated using structural magnetic resonance (MR). Although the largest body of literature exists for Alzheimer’s dementia, volumetric studies in DLB have helped in defining some of the neurobiological underpinnings of the disease, as well as making important contributions to the differential diagnosis of the condition and providing insights into its longitudinal progression. Analysis of structural data has included several methods ranging from simple visual inspection, through to region of interest (ROI) analysis and voxel-based morphometry (VBM). ROI and visual rating methods have been very much human led with operator-defined structures of interest, whereas VBM analysis has no a priori biases and makes statistical comparisons on a point-by-point basis (voxel level) across data sets.
Atrophic Changes in DLB
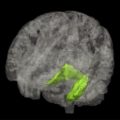
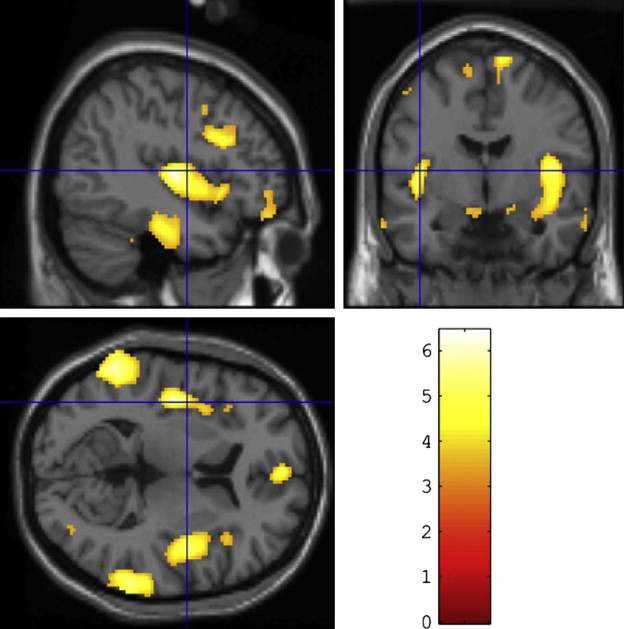
One of the main findings in AD is that of widespread global atrophy. In DLB the findings are less consistent; several studies have reported increased atrophy in DLB ( Fig. 1 ) in insular, frontal, inferior parietal, temporal, and occipital cortices compared with similarly aged controls, with relative sparing of the sensorimotor cortex. A large study by Whitwell and colleagues found very little cortical atrophy in patients with DLB aside from some subtle cortical loss in frontal and parietal lobes as well as volumetric reductions in hypothalamus, basal forebrain, and midbrain regions. The latter findings have also been reported in DLB by other groups, and may explain the marked cholinergic deficits apparent in DLB given that the nucleus basalis of Meynert is contained in the substantia innominata. However, in the studies by Whitwell and colleagues and Hanyu and colleagues, midbrain atrophy was also noted in AD patients, so these changes may not be specific to DLB.
Putamen atrophy has been reported in DLB in comparison with AD by Cousins and colleagues, although no significant structural differences have been found in the caudate nucleus of DLB patients in contrast to PDD, where atrophy of the right caudate tail was found in one structural VBM study. Overall, striatal structural atrophic changes may reflect increased α-synuclein pathology but they probably are less indicative of functional dopaminergic loss in DLB, probably for 2 main reasons. First, Cousins and colleagues did not find a link between striatal atrophy and the severity of motor symptoms, and second, the magnitude of atrophy (5%–10%) is much less marked than the large (50%) reductions that occur in DLB in the uptake of radioligands, which bind to the striatal dopamine transporter (see later discussion).
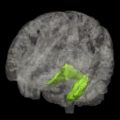
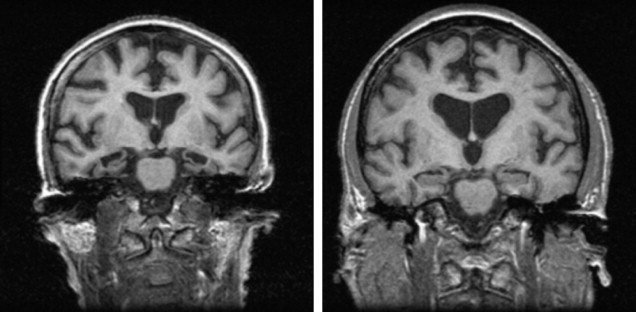
Overall, therefore, global and subcortical atrophic changes have not proved to be particularly useful in the differential diagnosis of DLB. Perhaps the most consistent finding has been the lack of medial temporal lobe atrophy in DLB compared with AD on both visual inspection ( Fig. 2 ) and volumetric analysis, although the presence of medial temporal atrophy does not rule out a diagnosis of DLB. Instead medial temporal lobe atrophy, if present, tends to rule in AD rather than other dementias; this is supported by an autopsy study of 46 patients with dementia (AD, DLB, and vascular) by Burton and colleagues, who noted that antemortem MR of the medial temporal lobe was robustly associated with Alzheimer tangle pathology rather than plaque or LB pathology within the temporal lobe.
Nevertheless, more recent reports using high-resolution structural MR imaging have suggested that subtleties exist between AD and DLB with regard to hippocampal subfield atrophy. Sabattoli and colleagues noted differential atrophy of more anterior regions in DLB compared with AD, consistent with CA2 and CA3 atrophy, and Firbank and colleagues reported reduced subiculum thickness, selective CA1 neuronal loss, and loss of distinction in a hypointense line that is visible between CA1 and CA3/4 in AD subjects in comparison with controls. This evidence is preliminary, but if replicated in larger samples high-resolution hippocampal imaging may become an additional tool in making a differential diagnosis between AD and DLB.
Progression of Atrophy in DLB
Several longitudinal studies using serial MR have suggested annual rates of atrophy that are comparable in patients with DLB and AD (approximately 1.5%–2% per year vs 0.5% in controls). Nevertheless, the presence of concomitant AD pathology may influence the trajectory of atrophic progression. In the study by Whitwell and colleagues, patients with pure AD, pure DLB, and mixed AD/DLB pathology were examined; patients with pure DLB had atrophy and ventricular expansion rates comparable with those of aged controls (0.4% and 4.8% per year, respectively) and were lower than subjects with AD (atrophy 1.1% per year with a ventricular expansion rate of 8.3% per year), whereas the mixed AD/DLB group had atrophy levels comparable with those of the AD group (1.3% per year, ventricular expansion 7.2% per year).
Atrophy and Association with Underlying Neuropathology and Clinical Symptoms
Structural MR imaging has also been used in DLB to help understand the relationship between the underlying neuropathology and associated neuropsychiatric and cognitive symptoms, although specific studies examining neuropathologic correlates of atrophy in DLB are relatively sparse. One major neuropathologic study with antemortem structural MR data by Burton and colleagues noted that a significant inverse correlation was observed between normalized amygdala volume and percent area of LBs in the amygdala, although there were no other significant correlations between regional volume and measures of neuropathology.
Cognitive symptoms in DLB may correlate with regional atrophic changes; for example, a comparative VBM study by Sanchez-Castaneda and colleagues of patients with DLB and PDD noted that patients with DLB had greater gray matter loss in frontal areas compared with patients with PDD, and that in patients with DLB prefrontal and anterior cingulate volume correlated with performance scores on a continuous performance test, a measure of sustained attention, whereas right hippocampal and amygdala volume was associated with visual memory function. Hippocampal atrophy (specifically CA1 volume reduction) in DLB was also found to be associated with poor memory function. These hippocampal data suggest that concomitant AD pathology may have a role in DLB in mediating memory dysfunction.
Given the marked visuoperceptual deficits and visual hallucinations that occur in DLB, others have sought to find regional atrophic changes, such as in the occipital lobe, that may relate to these clinical features. Occipital hypometabolism and hypoperfusion in DLB (see later discussion) have been observed using PET and SPECT imaging in DLB, but most volumetric studies using ROI analysis and VBM have failed to find any significant occipital structural changes in DLB, although one study using VBM did find more atrophy in the left occipital gyrus in patients with DLB than in controls. Overall, the lack of major occipital atrophy is probably consistent with the lack of significant LB pathology in the occipital pole. Other areas relating to higher visual processing and executive function may be more relevant for visual hallucinations; one recent report noted decreased volume in associative visual areas and the inferior frontal lobe, which correlated with visual hallucinations in DLB.
White Matter Hyperintensities
On T2-weighted and fluid-attenuated inversion recovery (FLAIR) images, white matter lesions (WML) or hyperintensities have been observed in patients with neurodegenerative disease and in healthy older people; pathologically these lesions relate to loss of myelin, axonal damage, and gliosis. Their presence often concords with small vessel disease, and it is presumed that they are a radiologic marker for localized vascular damage. However, the location of these lesions may be relevant; periventricular hyperintensities as opposed to deep white matter lesions correlate with increasing ventricular dilatation in patients with DLB and AD suggesting that, in these cases, the periventricular lesions relate more to atrophy than to vascular ischemia. Nevertheless, findings with regard to WML severity and their occurrence in DLB relative to controls and other neurodegenerative disorders have been inconsistent, and may relate to the analysis method used. Studies using visual rating scales have suggested that WMLs are more extensive in patients with DLB than in control subjects, although levels are similar to that of AD. However, another study that used automated quantification methods found no significant difference between the amount of WML in patients with DLB compared with controls and patients with PDD, although both groups had lesser amounts compared with patients with AD. These findings and the current lack of effects of WML on cognitive function in DLB mean that the role and importance of WML in the etiopathology of DLB remains to be resolved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree