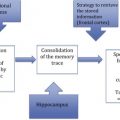
Reserve refers to the brain’s ability to cope with increasing damage. There is no direct measure of reserve, but it is commonly reflected in the literature by proxies such as brain volume, head size, education, occupation, socioeconomic status, and mental and physical engagement. This article provides an overview of the concepts and applications being used to explore reserve, and discusses how these empiric proxies of reserve, their hypothesized biological mechanisms, and the apparent protection from age-related disease are connected.
Reserve is a heuristic concept used to explain the apparent discrepancy between pathologic changes in the brain normally associated with aging and disease, and the manifestation of those changes in terms of clinical presentation and cognitive decline. It has been used to explain this discrepancy in a range of situations such as multiple sclerosis, dementia, and individual differences in cognitive aging. It is not the intention of this article to document an anthology of research concerned directly or indirectly with reserve, but to provide an overview of the concepts and applications being used to explore reserve and proposed connections between empiric proxies of reserve, hypothesized biological mechanisms, and the protection from aging and disease that reserve imparts.
A brief history of reserve
Interest in reserve has steadily increased since the early 1990s, with a Web of Science search identifying 138 publications using the term (or similar) in 2010 and more than 1200 publications since 1990. One of most frequently cited publications that refers to reserve is by Katzman and colleagues in 1988, who noted that those individuals with high levels of Alzheimer’s disease (AD) pathology post mortem, who otherwise remained nondemented in life, had almost double the number of large pyramidal neurons throughout their neocortex in comparison with those who expressed clinical symptoms. This publication goes on to suggest that those nondemented in life might have started with a larger brain and more neurons and thus might be said to possess greater reserve against incipient AD, but did not become clinically demented because of this greater reserve.
Although this is a modern articulation, the concept of brain reserve is not new. In the 1960s work done in and around Newcastle upon Tyne in the United Kingdom noted “it would appear that a certain amount of the change estimated by plaque counts may be accommodated within the reserve capacity of the cerebrum without causing manifest intellectual impairment,” and that “although the association between dementia and brain degeneration is impressive the extent of the degeneration varies considerably; moreover, occasionally, the brains of individuals who have never become demented have been found to show quite marked changes.” Moreover, “the condition of the brain seems therefore to be only one of several factors determining the threshold at which dementia appears.” Collectively these publications make two clear points: (1) the brain has a buffer or reserve capacity to withstand a degree of change brought about by aging and disease, and (2) the size of that capacity is different between individuals.
Although less scientifically vigorous, publications from the early twentieth century discuss the concept of reserve in mediating behavior in the face of pathologic change. Pickworth in the 1930s comments that “no clinical abnormality is noticed unless the damage is quantitatively so great as to exceed the reserve.” Indeed, Flemming in the 1920s goes as far as to suggest how reserve is acquired. “…The great storehouse for personal memories, the residue of individual experience. These reserves are drawn on to cooperate in deciding, on the basis of personal as well as racial experience, what act is appropriate to the situation.” These early publications are by no means the only two to articulate the concept of reserve in the early twentieth century.
With the increased availability of modern imaging techniques, the discrepancy between brain changes characteristic of disease and aging and an individual’s clinical presentation or cognitive performance has been reemphasized. The advent of specific β-amyloid (Aβ) ligands for positron emission tomography (PET), such as Pittsburgh compound B (PIB), has brought about great optimism for a new era in AD imaging diagnosis. However, not all patients with positive PIB PET have or develop AD. This fact may be attributable to the lack of specificity of the ligand and/or the exact role of Aβ in the development of AD, but it is also consistent with the notion that individual differences in reserve allow some people to maintain function in the face of disease-like pathologic burden. Similarly, a study using 18 F-fluorodeoxyglucose (FDG) PET indicated that some individuals (those with more years of schooling) were able to maintain function in the face of similar metabolic deficits. Stern and colleagues found that AD patients with more education had larger regional cerebral blood flow (rCBF) deficits, measured using single-photon emission computed tomography (SPECT), suggesting that more education enabled an individual to cope with greater levels of pathologic change. Reed and colleagues showed that schooling attenuates the cognitive effect of brain atrophy measured using magnetic resonance (MR) imaging.
Reserve models
It is unclear how reserve enables an individual to withstand the effects of age and disease-related pathologic change. Stern has suggested conceptual models for reserve and postulates different mechanisms for the implementation of reserve; the first he refers to as “brain reserve,” suggesting that individuals have different amounts of brain reserve and that, for a given amount of pathologic burden, those with more reserve will be less affected. This brain reserve could, therefore, be said to passively protect an individual against the effect aging and disease. Structural proxies of passive brain reserve suggested in the literature are indices such as brain or head volume, head circumference, synaptic count, or dendritic branching.
An active mechanism through which function is maintained has also been proposed, which has been described as cognitive reserve. An individual with this type of reserve uses brain networks or cognitive paradigms that are less susceptible to disruption and could be said to have “neural reserve.” The second mechanism is described as neural compensation. Here, in the face of pathology, the brain recruits structures and/or networks not normally used by individuals with intact brains, to compensate for pathologic burden. The literature has consistently identified proxies of active or cognitive reserve, such as premorbid cognitive ability, education, physical activity, intellectual engagement, and occupational attainment. These insightful models have structured reserve research and have provided a vocabulary with which to discuss the phenomenon.
These models may not be as discrete as they first appear and are not mutually exclusive. When examining brain reserve proxies, it is clear from the literature that these measures have also been shown to be modifiable through intervention and the environment. For example, McEwen has suggested that repeated stressors result in decreased dendritic branching and a reduction in the number of neurons. It has recently been suggested that early-life experience has a significant association with late-life brain size and may also be considered as a lifelong proxy of cognitive reserve. Therefore, brain reserve may represent, at least in part, the integration of life experience and environmental factors that overlap with the proxies of cognitive reserve. For example, a common proxy for cognitive reserve is education. Education is not only associated with premorbid ability but is influenced by childhood socioeconomic status, which in turn may reflect individual differences in diet and early-life stimulation. Moreover, education may influence late-life socioeconomic status and intellectual engagement, which may have an impact through passive and/or active routes. These structural, probably causal, paths between potential measures of reserve that extend across all models would suggest that reserve is unlikely to be optimally described by a single factor that can be measured directly.
Brain Reserve and the Passive Model
The passive reserve model can be summarized by the phrase “the more you have the more you have to lose before deficits appear.” “Having more” brain reserve, such as more synapses, will lead to sufficient residual capacity after accounting for pathology for function to continue unaltered. Insufficient reserve will lead to inadequate capacity, and functional loss will occur. There is considerable observational evidence that is consistent with the passive reserve model. Studies have reported higher levels of reserve proxies, such as brain size, neuronal number, and neuronal density in individuals with brain changes associated with dementia and disease but little or no cognitive deficits.
Cognitive Reserve and the Active Model
The active reserve model proposes that individual differences in processing mechanisms and the ability to compensate by actively adapting these mechanisms (and using new ones) is responsible for protecting an individual against the detrimental cognitive effects of aging and disease. A person’s premorbid intelligence as well as educational and occupational attainment have been suggested as proxies for measuring this ability. These measures are assumed to reflect the neural efficiency, or the ability to recruit alternative brain networks to compensate, when faced with pathology. Epidemiologic evidence supports the active or cognitive reserve hypothesis, with lower baseline intelligence scores, less education, and lower occupational attainment being risk factors for dementia and cognitive decline.
A processing mechanism that is less susceptible to pathology is a potential source of cognitive reserve. Consider two individuals who perform identically through different neuronal routes because they have different brain structures or different expertise and training, or who might have used different cognitive strategies. One may be considered to have reserve if the mechanisms used are less susceptible to aging and disease. Evidence for individual differences such as these has been considered in a review article by Deary and colleagues. An alternative source of active reserve has its origins in an individual’s ability to compensate for detrimental pathology. This reserve could be realized by upregulation of an existing mechanism (working harder) or by the recruitment of alternative or more extensive neural networks (doing different). A recent review by Eyler and colleagues into the functional brain imaging correlates of successful cognitive aging reported “frequent support for the notion that increased brain responsiveness is associated with better cognitive performance” in aging samples. However, it is unclear whether this increased responsiveness is a consequence of the better performers’ mechanisms of working harder or doing different. or whether better performers had increased responsiveness before the onset of pathologic burden and established mechanisms that are less susceptible to disease and aging. Or is it the case that a combination of all possible sources and mechanisms of reserve is required to cope with the onset of disease and aging? It is difficult to test for the presence of one strategy versus another using a cross-sectional, observational approach predominantly used in the field. For example; Persson and colleagues compared the frontal cortex functional (fMR) imaging response during a verbal encoding task between those who had stable memory performance and those who had declined. Greater response was found in the right ventral prefrontal cortex among decliners than in those with stable performance. Is this evidence of a declining person working harder or are those that develop more efficient mechanisms being less susceptible? Cabeza and colleagues found that young adults and poorer performing older adults recruited similar right prefrontal cortex (PFC) regions, whereas higher performing older adults engaged PFC regions bilaterally. The investigators concluded that poorer performing older adults recruit a network of brain regions similar to those of young adults, but use these inefficiently, whereas higher performing older adults compensate for age-related neural decline by reorganizing brain functions, that is to say, doing different in that face of aging. An alternative explanation would be that higher performing adults develop this alternative mechanism before the onset of aging pathology and are therefore less susceptible.
Reserve models
It is unclear how reserve enables an individual to withstand the effects of age and disease-related pathologic change. Stern has suggested conceptual models for reserve and postulates different mechanisms for the implementation of reserve; the first he refers to as “brain reserve,” suggesting that individuals have different amounts of brain reserve and that, for a given amount of pathologic burden, those with more reserve will be less affected. This brain reserve could, therefore, be said to passively protect an individual against the effect aging and disease. Structural proxies of passive brain reserve suggested in the literature are indices such as brain or head volume, head circumference, synaptic count, or dendritic branching.
An active mechanism through which function is maintained has also been proposed, which has been described as cognitive reserve. An individual with this type of reserve uses brain networks or cognitive paradigms that are less susceptible to disruption and could be said to have “neural reserve.” The second mechanism is described as neural compensation. Here, in the face of pathology, the brain recruits structures and/or networks not normally used by individuals with intact brains, to compensate for pathologic burden. The literature has consistently identified proxies of active or cognitive reserve, such as premorbid cognitive ability, education, physical activity, intellectual engagement, and occupational attainment. These insightful models have structured reserve research and have provided a vocabulary with which to discuss the phenomenon.
These models may not be as discrete as they first appear and are not mutually exclusive. When examining brain reserve proxies, it is clear from the literature that these measures have also been shown to be modifiable through intervention and the environment. For example, McEwen has suggested that repeated stressors result in decreased dendritic branching and a reduction in the number of neurons. It has recently been suggested that early-life experience has a significant association with late-life brain size and may also be considered as a lifelong proxy of cognitive reserve. Therefore, brain reserve may represent, at least in part, the integration of life experience and environmental factors that overlap with the proxies of cognitive reserve. For example, a common proxy for cognitive reserve is education. Education is not only associated with premorbid ability but is influenced by childhood socioeconomic status, which in turn may reflect individual differences in diet and early-life stimulation. Moreover, education may influence late-life socioeconomic status and intellectual engagement, which may have an impact through passive and/or active routes. These structural, probably causal, paths between potential measures of reserve that extend across all models would suggest that reserve is unlikely to be optimally described by a single factor that can be measured directly.
Brain Reserve and the Passive Model
The passive reserve model can be summarized by the phrase “the more you have the more you have to lose before deficits appear.” “Having more” brain reserve, such as more synapses, will lead to sufficient residual capacity after accounting for pathology for function to continue unaltered. Insufficient reserve will lead to inadequate capacity, and functional loss will occur. There is considerable observational evidence that is consistent with the passive reserve model. Studies have reported higher levels of reserve proxies, such as brain size, neuronal number, and neuronal density in individuals with brain changes associated with dementia and disease but little or no cognitive deficits.
Cognitive Reserve and the Active Model
The active reserve model proposes that individual differences in processing mechanisms and the ability to compensate by actively adapting these mechanisms (and using new ones) is responsible for protecting an individual against the detrimental cognitive effects of aging and disease. A person’s premorbid intelligence as well as educational and occupational attainment have been suggested as proxies for measuring this ability. These measures are assumed to reflect the neural efficiency, or the ability to recruit alternative brain networks to compensate, when faced with pathology. Epidemiologic evidence supports the active or cognitive reserve hypothesis, with lower baseline intelligence scores, less education, and lower occupational attainment being risk factors for dementia and cognitive decline.
A processing mechanism that is less susceptible to pathology is a potential source of cognitive reserve. Consider two individuals who perform identically through different neuronal routes because they have different brain structures or different expertise and training, or who might have used different cognitive strategies. One may be considered to have reserve if the mechanisms used are less susceptible to aging and disease. Evidence for individual differences such as these has been considered in a review article by Deary and colleagues. An alternative source of active reserve has its origins in an individual’s ability to compensate for detrimental pathology. This reserve could be realized by upregulation of an existing mechanism (working harder) or by the recruitment of alternative or more extensive neural networks (doing different). A recent review by Eyler and colleagues into the functional brain imaging correlates of successful cognitive aging reported “frequent support for the notion that increased brain responsiveness is associated with better cognitive performance” in aging samples. However, it is unclear whether this increased responsiveness is a consequence of the better performers’ mechanisms of working harder or doing different. or whether better performers had increased responsiveness before the onset of pathologic burden and established mechanisms that are less susceptible to disease and aging. Or is it the case that a combination of all possible sources and mechanisms of reserve is required to cope with the onset of disease and aging? It is difficult to test for the presence of one strategy versus another using a cross-sectional, observational approach predominantly used in the field. For example; Persson and colleagues compared the frontal cortex functional (fMR) imaging response during a verbal encoding task between those who had stable memory performance and those who had declined. Greater response was found in the right ventral prefrontal cortex among decliners than in those with stable performance. Is this evidence of a declining person working harder or are those that develop more efficient mechanisms being less susceptible? Cabeza and colleagues found that young adults and poorer performing older adults recruited similar right prefrontal cortex (PFC) regions, whereas higher performing older adults engaged PFC regions bilaterally. The investigators concluded that poorer performing older adults recruit a network of brain regions similar to those of young adults, but use these inefficiently, whereas higher performing older adults compensate for age-related neural decline by reorganizing brain functions, that is to say, doing different in that face of aging. An alternative explanation would be that higher performing adults develop this alternative mechanism before the onset of aging pathology and are therefore less susceptible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree