2.2 Osteolytic Lesions and Lesions with Mixed Features
2.2.1 Case 8 (Fig. 2.15)

Case description
Referring physician: radiologist.
Prior history and clinical question: A 58-year-old woman, otherwise healthy, claimed to have experienced an occasional warm sensation predominantly in her left forehead region over a long period of time. She stated that her son, who lived abroad, had noticed that her head appeared enlarged. Later she saw her family doctor, who drew blood samples. The tests showed a two-fold elevation of alkaline phosphatase levels, but her doctor was unsure how to proceed. Finally her son insisted that she have her head imaged. The radiologist recommended that we should narrow the differential diagnosis between Paget disease and fibrous dysplasia. His practice is in southern Germany, where doctors have had relatively little experience with Paget disease. (This is absolutely correct, because Paget disease is rare in southern Germany but almost endemic in northern Germany, occurring mainly in older individuals.)
Radiologic Findings
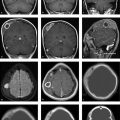
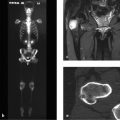
The lateral skull radiograph (Fig. 2.15 a) shows cloudy structural changes consisting of a mixture of lucencies and opacities. The radiologist correctly followed the radiographs with CT scans (Fig. 2.15 b), which show generalized thickening of the calvarium that is most pronounced in the left frontoparietal region. The normal three-layered structure of the calvarium has been almost completely lost. While irregular ossification is found in the diploë on the right side, the left frontoparietal region is hypodense and contains islands of ossification. Other hyperdense islands and lytic areas are noted farther posteriorly on the left side.
Location
The skull radiograph and CT show involvement of all three wall layers throughout the calvarium.
Pathoanatomic Background of the Findings
The imaging findings show that normal bony structures in the skull have been replaced by irregular ossifications that expand the skull volume. The images show an alternation of bone resorption and irregular new bone formation, suggesting that the process is still active.
Assignment to a Possible Basic Entity
Normal variant or malformation?
No. The remodeling process had its clinical onset in adulthood (note the history!). The elevated serum alkaline phosphatase level is also inconsistent with a normal variant.
Trauma?
No, the patient gave no history of trauma.
Inflammation?
Yes. Bone destruction and reparative new bone formation are typical features of bone inflammation. Common osteomyelitis is definitely excluded by the absence of clinical signs (local heat, pain, fistulation, etc.) and larger areas of bone destruction. Thus, we must be dealing with an inflammationlike disorder, and the only possibility is Paget disease (osteitis deformans).
Tumor?
No. The process is much too extensive and diffuse for a neoplasm.
Systemic disease (metabolic, reticulohistiocytosis, storage disease)?
No. Except for elevated serum alkaline phosphatase, there is no clinical or laboratory evidence for these conditions. A bone scan (not pictured here) showed intense uptake only in the skull, similar to Case 14. This would also exclude hyperparathyroidism (see Case 15).
Synopsis and Discussion
The coexisting signs of bone resorption and irregular reparative new bone formation (woven bone) with an expansion of cranial volume are hallmarks of the intermediate stage of Paget disease (see below under Intermediate or mixed stage). This diagnosis is also consistent with patient age, lesion location (the skull is the fourth most common site of occurrence after the pelvis, femur, and tibia), and elevated alkaline phosphatase. Note that if the volume of the changes were small, the osteoblast burden would be insufficient to raise the serum alkaline phosphatase level.
Paget disease is an inflammationlike disease of bone that progresses through three characteristic pathologic, clinical and radiologic stages and may involve one or multiple bones:
Lytic stage: The classic model starts with a lytic stage initiated by a viral infection stimulating osteoclastic activity. The osteoclasts cause a very rapid breakdown of bone, resulting in osteolysis (see Case 9 and Case 12).
Intermediate or mixed st age: Bone remodeling at this stage is marked by a reaction of osteoblasts and activated fibroblasts with the exuberant formation of new, nonlamellar, primitive woven bone.
Sclerotic stage: A predominance of bone formation over bone resorption leads over time to a dense network of calcified woven bone with a high fiber content, which creates irregular coarsened structures that are particularly characteristic of Paget disease in the pelvis and long bones.
Bone scintigraphy generally shows intense uptake in the early and intermediate stages. This is seen even on early images since the remodeling bone area has a rich blood supply and the mineralizing woven bone is very avid in its uptake of radiotracer. The onset of Paget disease may occur as early as the fourth decade. Since it usually runs an asymptomatic course, it often goes undiagnosed until the sixth to eighth decade when it is often detected incidentally, sometimes from an elevated serum alkaline phosphatase level.
Paget disease of the skull mainly requires differentiation from fibrous dysplasia. The main differentiating criterion is the ground-glass appearance on imaging, described fully in Case 4 and Case 7. Moreover, patients with fibrous dysplasia are younger when the change is first detected. The changes in Paget disease are generally symmetrical. Fibrous dysplasia tends to spare the cranial sutures. Sites of predilection for fibrous dysplasia are the sphenoid bone and paranasal sinuses, while Paget disease most commonly affects the calvarium. The outer and inner tables are typically thickened in Paget disease; this finding is less pronounced in fibrous dysplasia.
When the radiologic changes in the skull are considered in isolation, the differential diagnosis would also have to include hyperparathyroidism (see Case 15), but this disease is excluded because in the latter abnormal tracer uptake is not limited to the skull, as it is in Paget disease. It is sometimes difficult histologically to distinguish between Paget disease and hyperparathyroidism.
Final Diagnosis
Mixed (intermediate) stage of Paget disease.
Comments
Lytic lesions in the calvarium coexisting with irregular new bone formation and volume expansion are strongly suggestive of Paget disease when found in an older, otherwise healthy patient. An elevated serum alkaline phosphatase level can further aid the diagnosis, although this value will not be elevated if the structural changes have affected only a small bone volume.
2.2.2 Case 9 (Fig. 2.16)

Case description
Referring physician: radiologist.
Prior history and clinical question: A routine checkup in a 76-year-old man revealed an elevated serum alkaline phosphatase level. Subsequent whole-body bone scintigraphy (not shown) demonstrated focal areas of intense uptake in the calvarium. Because the radiograph showed large osteolytic areas, a metastatic tumor was suspected. This prompted comprehensive inpatient screening for a primary tumor, which was unsuccessful. Afterward we were asked to perform a percutaneous biopsy. Based on the geographic pattern of the osteolytic areas and absence of clinical abnormalities (e.g., palpable soft-tissue masses over the lytic areas), we doubted the previous diagnosis of metastases and suspected osteoporosis circumscripta. A subsequent cranial CT examination confirmed our suspicion.
Radiologic Findings
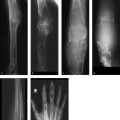
The lateral skull radiograph (Fig. 2.16 a) demonstrates large geographic lucencies in the parietal region, consistent with scintigraphic findings (not shown). Axial CT (Fig. 2.16 b) shows a long demineralized area in the parietal bone with an intact basic architecture of the bone.
Location
Parietal region, involving all layers of the calvarium.
Pathoanatomic Background of the Findings
The process in this case is associated with a loss of bone substance without signs of osteolytic destruction. The elevated alkaline phosphatase level also indicates concomitant bone formation. Overall, however, the process is predominantly catabolic.
Assignment to a Possible Basic Entity
Normal variant or malformation?
No. Circumscribed “atrophic” processes that are associated with decreased bone density and a normal bone shape are not known to occur as normal variants in the calvarium (compare with Case 10 and Case 13).
Trauma?
Negative history.
Inflammation?
Certainly not in the traditional sense, given the absence of true destructive and reparative elements and the absence of sequestra on CT. On the other hand, a very early stage of Paget disease presents as an inflammationlike condition marked by osteoclastic resorption, known also as osteoporosis circumscripta (see below under Synopsis and Discussion). This early “osteolytic” stage starts with multiple small foci in the skull and may last for years. Osteoblastic and fibroblastic areas with fiber bone formation are detectable microscopically at this stage but not radiologically (see Case 8 for further pathoanatomic details).
Tumor?
No. It would be most unusual for a destructive neoplastic process to leave the shape and basic architecture of the bone intact while tumor cells simply “demineralized” the affected bone area.
Perfusion disorder?
Hyperperfusion due to a trophic disturbance with subsequent demineralization (transient edema followed by transient osteoporosis) has not yet been observed at this location. Sites of predilection for transient osteoporosis are juxta-articular bone areas about the hip, knee, ankle, and subtalar joints.
Synopsis and Discussion
The descriptive term osteoporosis circumscripta (see Inflammation? above) is reserved exclusively for the early osteolytic stage of Paget disease. If the radiologist notes circumscribed demineralization of the cranial vault without destructive changes on radiographs, and then proceeds with CT to confirm the nondestructive character of the lesion(s), this process in itself will supply the correct diagnosis—without histologic confirmation. The determination of serum alkaline phosphatase may also be helpful.
In this case it would be unrealistic to include metastasis in the differential diagnosis.
Eosinophilic granuloma (focal Langerhans cell histiocytosis, Fig. 2.17) is also highly unlikely because it is a disease of preschool and school-age children. To date we have observed a few isolated cases in the sixth and seventh decades and have seen none in the eighth decade. The child in Fig. 2.17 was asymptomatic. The temporal bone lesion enlarged noticeably over a 1-year period and was joined by a second lesion in the occipital bone. In this case there is no reasonable alternative to a diagnosis of eosinophilic granuloma.

Final Diagnosis
Early lytic stage of Paget disease.
Comments
Remember: not every osteolytic lesion on radiographs or CT scans is a metastasis. There are other diseases! Metastasis should be diagnosed sparingly, as it can be very distressful for an otherwise healthy patient.
2.2.3 Case 10 (Fig. 2.18)

Case description
Referring physician: oncologist.
Prior history and clinical question: A 68-year-old woman presented with recurrent episodes of occipital pain and swelling. A previous imaging examination was interpreted as showing areas of occipital bone destruction suspicious for bone metastasis. A primary tumor is not known.
Radiologic Findings (Primary)
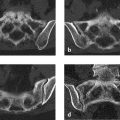
The projection radiograph (Fig. 2.18 a) demonstrates three well-circumscribed osteolytic areas that are “cold” on scintigraphy (Fig. 2.18 c). CT displays the areas as smoothly marginated defects with paper-thin outer and inner tables (Fig. 2.18 b). No paraosseous component is seen.
Location
The epicenter of the lesions is in the diploë.
Pathoanatomic Background of the Findings
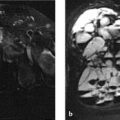
It is important to ask what is occupying the defects in place of bone tissue and diploë (fluid, fat, connective tissue?). MRI is the best modality for distinguishing between connective tissue and fluid. T2-weighted (T2w) image slices through the defects (Fig. 2.18 d) show a structure that is isointense to cerebrospinal fluid (CSF). To determine whether this is actually CSF, a FLAIR (fluid-attenuated inversion recovery) sequence is acquired which suppresses the signal from CSF. This sequence (Fig. 2.18 e) shows an absence of signal from the CSF spaces and the defects, confirming that the defects are occupied by CSF-filled arachnoid diverticula.
Assignment to a Possible Basic Entity
Normal variant or malformation?
Yes. Arachnoid diverticula are among the most familiar normal variants in the cranial vault. They are also called Pacchionian granulations or arachnoid villi. CSF is reabsorbed by intradural arachnoid villi or Pacchionian granulations and drains into the dural sinuses. These arachnoid villi can form pitlike recesses of variable size (10 mm to several centimeters) in the inner table and diploë, which may cause extreme thinning of the outer table with an associated watch-glass bulge. The most common sites of occurrence are the frontal bone and parietal bone. The granulations are distributed along major venous sinuses, so it is helpful to check the proximity of the diverticula to vascular channels on the images.
Trauma?
No history.
Inflammation?
No clinical manifestations.
Tumor?
This is the main entity requiring differentiation from large arachnoid diverticula on plain radiographs. The most likely possibilities are eosinophilic granuloma, multiple myeloma, and metastases. The negative bone scan virtually excludes metastases but does not exclude multiple myeloma. It would be unusual for metastasis or multiple myeloma to affect only one area of the cranial vault—in this case the occipital bone. This also applies to eosinophilic granuloma (focal Langerhans cell histiocytosis, see Fig. 2.17 in Case 9). The latter diagnosis is also inconsistent with the advanced age of the patient. In any case, the information supplied by the FLAIR sequence is sufficient to exclude these tumors.
Synopsis and Discussion
Normal variants, especially Pacchionian granulations, should be considered whenever osteolytic areas are detected radiographically in the cranial vault. This is particularly true when the changes are confined, atypically, to one cranial bone. If subsequent CT scans demonstrate smooth-bordered masses with no paraosseous spread or adjacent reactive sclerosis, a normal variant is even more likely. Because the patient in this case had complaints which prompted diagnostic imaging, it is reasonable to proceed with further imaging studies; the first-line option is MRI with a FLAIR sequence. If this modality is not available, bone scintigraphy is a second-line option which, when negative, will at least make metastasis a very unlikely diagnosis.
We cannot explain the clinical symptoms (recurrent occipital pain and swelling) that this patient manifested in connection with Pacchionian granulations or arachnoid diverticula.20 Cases published to date in the literature have described similar symptoms, for which no plausible explanation has been found.
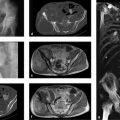
To aid the diagnostician in differentiation from Pacchionian granulations, Fig. 2.19 illustrates the case of a 46-year-old woman with an intraosseous epidermoid (synonyms: epithelial cyst, epidermoid cyst in bone) in the left occipital area. Whole-body bone scans in this patient demonstrated slight uptake in the left occipital area. Because the patient had breast cancer, further imaging studies were performed. The CT scans in Fig. 2.19 a–f show a large, central osteolytic lesion in the calvarium with smooth margins and slight internal expansion. The lesion is covered partly by a very thin remnant of the inner and outer tables, producing a watch-glass bulge, and partly by periosteum alone. There is no apparent infiltration of adjacent structures. The attenuation values in the lesion range from 10 to 50 HU. These images are almost identical to the one in Fig. 2.18 b. On T1w MRI (Fig. 2.19 g) the lesion is slightly hyperintense to cerebellum and shows only faint enhancement after contrast administration (Fig. 2.19 h). In the T2w image (Fig. 2.19 i) the lesion exhibits an outer proton-rich zone and an inner or central hypointense zone. The CSF-suppressed FLAIR sequence in Fig. 2.19 j identifies the outer hyperintense zone in image Fig. 2.19 h as fluid. Further crucial information is supplied by the diffusion-weighted EPI (echo planar imaging) sequence (Fig. 2.19 k, l) and the ADC (apparent diffusion coefficient) image (Fig. 2.19 m). The lesion appears hyperintense in the EPI sequence. The ADC image shows that the high signal intensity in Fig. 2.19 k, l is caused by a diffusion abnormality and not by T2w shine-through effects. Taken with the clinical presentation (incidental discovery), these findings are strongly suggestive of an intraosseous epidermoid. This case is very similar to one published by Dammert et al. (2003),21 who stated that the diffusion abnormality documented by diffusion-weighted imaging and the ADC image is highly specific for an epidermoid. An intraosseous epidermoid develops when epidermis becomes displaced beneath the periosteum or into the bone. The active germinal matrix produces hyperkeratosis as well as para- and dyskeratosis. This creates an osteoclastic stimulus leading to local bone resorption. The epidermal tissue may become misplaced as a result of trauma, but a dysontogenetic origin is thought to be more likely in the skull. Histologically, the cyst wall is formed by a uniform layer of squamous epithelium that develops a granular cell layer on the luminal side as it matures. It is covered externally by a thin fibrotic layer. The cyst lumen is lined by desquamated and compressed keratin lamellae.

Another epidermoid case, illustrated in Fig. 2.20, confirms the claim of Dammert et al. This 18-year-old patient had struck the right side of her head on a bar while playing sports at school. Afterward she noticed an indentation in the right posterior parietal area, which subsequently enlarged, so she and her parents claimed. She was diagnosed clinically with a minor head injury, which was not thought to be related to the indentation. CT, which was not performed until 3 months later, showed a full-thickness bone defect in the right posterior parietal area (Fig. 2.20 a–c). The differential diagnosis consisted of eosinophilic granuloma and epidermoid cyst. On T1w MRI (Fig. 2.20 d) the lesion appears hypointense to brain and slightly hyperintense to bone. It shows only a slight degree of peripheral enhancement (Fig. 2.20 e). In the T2w image (Fig. 2.20 f) the lesion is isointense to CSF, and in the FLAIR sequence (Fig. 2.20 g) it is isointense to brain. Finally, the lesion is isointense to brain structures in the ADC images (Fig. 2.20 h). All of the findings are consistent with an intraosseous epidermoid. Because the patient was asymptomatic—and it is ultimately unknown whether eosinophilic granulomas may pass through stages in which they resemble an intraosseous epidermoid on MRI—we recommended surgical excision. Histology at 7 months after the traumatic event identified the lesion as an epidermoid cyst with a focal granulating and fibrotic inflammatory reaction, interpreted partly as a foreign-body reaction.

This last case naturally raises the question of whether the head trauma induced the epidermoid cyst, as the clinical presentation suggests. But epidermoid cysts or intraosseous epidermoids in the calvarium are generally asymptomatic. The young woman first noticed a depression in the right calvarium after the impact trauma and claimed that the palpable depression had enlarged over time. But this would have been possible only if the skin over the lesion area had been disrupted, allowing epithelium to become displaced beneath the periosteum or directly into the bone through a fractured outer table. This would most likely have caused a hematoma with an associated bulge, not an indentation. We believe, rather, that the head impact ruptured a pre-existing cyst, thereby reducing its volume and forming a palpable depression in the skull. The histologic finding of an inflammatory foreign-body reaction suggests that the rupture had been present for some time. This case is quite interesting from a forensic standpoint as well.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree