3.2 Oligo- and Multisegmental Changes
3.2.1 Case 27 (Fig. 3.18)

Case description
Referring physician: orthopedist.
Prior history and clinical question: A 12-year-old girl presented with nonspecific posterior neck pain. A radiograph of her cervical spine showed calcifications in the C4–C5 and C5–C6 disk spaces and wedging of the C5 vertebra, which were considered suspicious for a tumor. The case was investigated further by MRI, but without a conclusive diagnosis.
Radiologic Findings
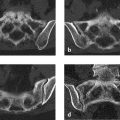
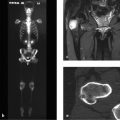
The lateral radiograph of the cervical spine (Fig. 3.18 a) shows disk-shaped calcifications in the C4–C5 and C5–C6 disk spaces with anterior wedging of the C5 vertebral body. The sagittal T2w image (Fig. 3.18 b) shows that the nucleus above C5 is shortened and tilted anteriorly. Its anterior tip points toward a defect devoid of MR signal. Other projections and sequences added no information, so it was decided to obtain low-dose CT scans of C4 through C6. The reformatted images in Fig. 3.18 c, d show a defect in the upper endplate of the wedged C5 vertebra, and below the defect is a space devoid of cancellous bone. Fig. 3.18 c also shows a small defect in the lower endplate of C5. Clumped calcifications are present in the C4–C5 and C5–C6 disk spaces. The larger calcification at C5–C6 is shaped like a synthetic intervertebral disk. Small flecks of calcification are visible within the defect in C5. The vertebral body height of C5 is significantly reduced.
Pathoanatomic Background of the Findings
The intervertebral disk calcifications, endplate defects, and the defect in C5 are obviously interrelated. Dense clumps of calcification generally result from a previous (old) necrotic process, here involving the nuclei pulposi. Partial necrosis of the anterior apophysis of C5 could account for its diminished height, and an intervertebral disk herniation could explain the defect in C5.
Assignment to a Possible Basic Entity
Normal variant or malformation?
Not known to occur in this form.
Trauma?
No history.
Inflammation?
Possibly; it would have to be an aseptic inflammation culminating in necrosis.
Tumor?
No, because where would the tumor be located? In the defect in C5? And how could the other findings be explained?
Perfusion defect with necrosis?
As noted under Pathoanatomic Background, necrosis could explain all the findings (see Synopsis and Discussion below), though it would have to represent the end stage of an ultimately unidentified disease process (inflammation, primary perforation disorder, trauma?).
Synopsis and Discussion
All the findings are consistent with a condition known in the literature as “calcifying discitis.” This entity is observed in children and adolescents but its etiology is poorly understood. Approximately 75% of affected children present clinically with pain, limited motion, and torticollis. Fever and leukocytosis have also been reported and suggest an inflammatory etiology.27 Some children also have a history of trauma. The cervical spine is a site of predilection. The calcifications may be transient, lasting for a period of weeks or months, or they may persist. Perforation of the calcium collections into the vertebral bodies, intervertebral foramina, spinal canal, or surrounding soft tissues has been described.
In the present case we can reconstruct the pathogenesis as follows: nonspecific, nonbacterial inflammation of the disk space structures at C4–C5 and C5–C6, followed by destruction of the intervertebral anatomic structures with necrosis and secondary calcification (necrotic tissue is a “calcium sink”), leading to pressure on the adjacent endplates causing rupture and intravertebral disk herniation.
Final Diagnosis
Calcifying discitis in the C4–C5 and C5–C6 disk spaces.
Comments
Structures in the intervertebral disk spaces that resemble synthetic disks may signify calcifying discitis.
3.2.2 Case 28 (Fig. 3.19)

Case description
Referring physician: orthopedist.
Prior history and clinical question: A 64-year-old colleague noticed increasing stiffness of the spine with an inability to bend his neck backward. He has gout and type 2 diabetes mellitus. He was concerned that he may have ankylosing spondylitis. He submitted a prior radiograph of the cervical spine taken 6 years previously, stating that the large spondylophyte visible on C5 was interpreted as a degenerative osteophyte in a setting of osteochondrosis.
Radiologic Findings
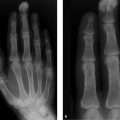
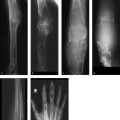
The cervical spine radiograph from 1986 (Fig. 3.19 a) shows a large spondylophyte projecting downward from the lower anterior half of C5. The corresponding disk space is of normal height. Another, more band-shaped ossification arises from the lower anterior half of C2. A radiograph taken in 1992 (Fig. 3.19 b) shows more pronounced, ankylosing (flowing, “sugar-coated”) ossifications from C2 to C7. All the disk spaces are of normal height, and the endplates show no abnormalities. A posteroanterior radiograph of T12–L3 taken in 1992 (Fig. 3.19 c) shows conspicuous, ankylosing ossifications along the sides of the vertebrae.
Location
The ossifications do not originate from the vertebral margins (diskovertebral junctions) but from the anterior and lateral ligamentous structures.
Pathoanatomic Background of the Findings
The ossifications are solid and apparently developed in the absence of previous or associated spinal disease. They originate from ligamentous structures.
Assignment to a Possible Basic Entity
Normal variant or malformation?
Both are excluded by the progression of the changes over time.
Trauma?
No history.
Inflammation?
No corresponding signs (e.g., destruction of vertebral structures, clinical findings).
Tumor?
No, there is no evidence of a “space-occupying” process.
Regressive process?
Very likely. This interpretation is supported by the age of the patient and the absence of any inflammatory signs.
Synopsis and Discussion
The hyperostotic changes on the outer surfaces of the affected vertebral bodies are characteristic of diffuse idiopathic skeletal hyperostosis (DISH), known also as hyperostotic spondylosis deformans or Forestier disease. The criteria for this diagnosis are as follows:
Flowing, ankylosing spondylophyte formation along the front and sides of at least four contiguous vertebrae
No signs of osteochondrosis
Additional criteria:
Ligament ossifications (e.g., posterior longitudinal ligament, pelvic ligaments)
Productive fibro-ostosis (proliferative enthesophathy)
Exclusion criteria:
Degenerative changes in intervertebral disks
Sacroiliitis
The etiology is unknown. There is a definite association with diabetes mellitus and gout, both of which have a certain “osteoplastic disposition.” The patient in this case had diabetes mellitus and gout. The hyperostosis is manifested chiefly in structures with dense connective tissue, especially ligaments. This distinguishes DISH from ordinary spondylosis deformans.
In any type of spondyloarthritis (ankylosing spondylitis, psoriatic spondyloarthritis, etc.), the ossifications (syndesmophytes, parasyndesmophytes) arise from the vertebral margins or diskoligamentous junctions, which represent entheses. Other enthesitic changes occur in the vertebral endplates, which are also entheses. In the florid stage the inflammatory changes are characterized by simultaneous bone destruction and proliferation. The sacroiliac synchondroses (“joints”) and/or manubriosternal synchondrosis are commonly affected, as are the capsular and ligamentous insertions (see also Case 39, Case 40, Case 48, Case 49, Case 84, Case 97, and Case 145).
Final Diagnosis
DISH.
Comments
DISH mainly requires differentiation from spondyloarthritis and ordinary osteochondrosis with spondylophyte formation. Noting the diagnostic criteria for DISH permits an accurate classification of the hyperostotic changes in the spine.
3.2.3 Case 29 (Fig. 3.20)


Case description
Referring physician: radiologist.
Prior history and clinical question: A 51-year-old woman had pain and increasing stiffness in her cervical spine, 1 year after sustaining a basal skull fracture. MRI of the cervical spine showed unusual paravertebral soft-tissue changes on the left side at the C3–C7 level in addition to vertebral changes (hypointense in T1w images, hyperintense in T2w and TIRM images) that could not be definitely classified. The next logical study, CT examination of the neck, yielded a surprising result.
Radiologic Findings
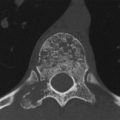
On MRI the C3–C5 vertebrae have low signal intensity in the T1w sequence (Fig. 3.20 a) and appear eroded anteriorly. An anteriorly convex mass, isointense to muscle, is visible along the anterior aspect of the vertebrae, extending inferiorly to the C7 level. The changes have high signal intensity in water-sensitive sequences (Fig. 3.20 b, c). The CT scans in Fig. 3.20 d–r show sclerosis in C3 and C4 and partial sclerosis in C5, which appears slightly eroded anteriorly. The vertebrae are bordered by an irregularly ossified mass that shows anterior and anterolateral extension on the left side. The mass is bordered in turn by a still-unossified soft-tissue mass that has displaced the left retropharyngeal structures anteriorly. Bone scintigraphy (Fig. 3.20 s) shows intense uptake in the left anterolateral portion of the cervical spine.
Location
The epicenter of the ossifications is located in the space between the anterior vertebral margins and anterior longitudinal ligament.
Pathoanatomic Background of the Findings
The findings indicate an ossifying or bone-forming process, still in a florid stage (positive bone scan), with associated erosion of the affected anterior vertebral contours and sclerosis of the underlying cancellous bone. Anterior to the ossifying process is a purely soft-tissue mass at a left anterolateral location.
Assignment to a Possible Basic Entity
Inflammation?
Possibly. Nonbacterial inflammatory changes in tendons often have significant fibroblastic potential. Trauma has been suggested as a precipitating cause for most cases. The histologic detection of inflammatory cells does not necessarily mean that a process has a primary inflammatory cause. Inflammatory cells are also found as an epiphenomenon following trauma, as in myositis ossificans.
Trauma?
The patient has a positive trauma history, having suffered a basal skull fracture a year earlier. There may have been trauma to pre- and paravertebral soft tissues inciting the development of myositis ossificans. Florid myositis ossificans presents a characteristic trizonal pattern (peripheral ossification followed by an intermediate zone, then an immature zone), which reflects the maturation of the process and is clearly apparent on the CT scans (Fig. 3.20 g–j). But this alone does not explain the unossified mass, unless we assume that myositis ossificans occurred as a two-stage process.
Tumor?
Periosteal osteosarcoma may invade the bone, but the portion near the bone ossifies first while the peripheral portion ossifies last (“reverse trizonal pattern”).
Synopsis and Discussion
There are three realistic differential diagnoses for the partly contradictory findings:
Myositis ossificans (heterotopic ossification) progressing in two stages
Periosteal (juxtacortical) or high-grade surface osteosarcoma
Prevertebral tendinitis
On differential diagnosis 1
The most frequent cause of myositis ossificans is trauma (traumatic myositis ossificans; see also Case 156). The patient was seen 1 year after sustaining a basal skull fracture, with the complaint of neck pain and increasing neck stiffness. It is likely that the trauma caused bleeding beneath the tough anterior longitudinal ligament, which precipitated the development of myositis ossificans. This could also explain the anterior convexity of the prevertebral ossifications. The density of the ossifications is consistent with a 1-year history of myositis ossificans. The pressure from the firm anterior longitudinal ligament led to erosions in the affected anterior vertebral margins and intracancellous sclerosis. It is also conceivable that a primary bony injury may have caused the anterior vertebral erosions and intraosseous sclerosis. It is not unusual for myositis ossificans to progress in two stages; we have repeatedly observed this in the pelvis and appendicular skeleton (see Case 156). This process is marked by a solid ossification with cancellous bone formation coexisting with a soft-tissue mass that has not yet ossified. The soft-tissue mass might also be caused by tendinitis of the prevertebral muscles developing as a result of anatomic changes from the myositis ossificans (see On differential diagnosis 3 below).
On differential diagnosis 2
Periosteal osteosarcomas grow on the bone surface. They develop predominantly in the adjacent soft tissues; infiltration of the medullary cavity is somewhat rare. Cases of this kind always raise the possibility of a high-grade surface osteosarcoma. The above-mentioned “reverse trizonal pattern” of ossifications in periosteal osteosarcoma is not found in the present case. The most common sites of occurrence of periosteal osteosarcoma are the femur and tibia. Uncommon sites are the ribs, maxilla, and pelvis. We know of no instances of vertebral involvement. Periosteal osteosarcoma accounts for only 1% of all osteosarcomas. When we consider probabilities in the diagnosis of borderline cases like this one, we must acknowledge that myositis ossificans after a basal skull fracture is a far more likely diagnosis than a rare periosteal osteosarcoma occurring at a previously unknown site. The same considerations apply to high-grade surface osteosarcoma, which is even rarer than periosteal osteosarcoma and most commonly involves the femur and tibia.
On differential diagnosis 3
Prevertebral tendinitis (also called retropharyngeal tendinitis) is an acute self-limiting disease of the longus colli muscle.28 , 29 Its etiology is unknown but may relate to crystal deposits with a secondary inflammatory response, trauma, hemorrhage, a primary idiopathic inflammation, or necrotic processes. The site of predilection is the upper cervical spine. Most patients with this very rare disease are in the fifth or sixth decade of life. Our case involves very pronounced ossifications and adjacent osseous changes that do not fit the usual presentation or location of prevertebral tendinitis. At most, we might refer the unossified mass to prevertebral tendinitis, considering it a result of chronic trauma to the prevertebral muscles due to anatomic changes (see above On differential diagnosis 1).
It is questionable whether a histologic examination would yield a definitive result. These processes are difficult to distinguish from one another by their histology. The lesion may very well contain areas with cellular atypias, increased mitosis, etc. that would be interpreted as malignant. Conversely, there are cases in which ossifications recurring over a period of years are classified histologically as myositis ossificans until a juxtacortical osteosarcoma is eventually identified. Due in part to difficult access, this case is still being followed while symptoms are managed with NSAIDs.
Working Diagnosis
Traumatic myositis ossificans for further observation.
Comments
Rare disease entities that are manifested at even rarer sites can often be identified only by long-term follow-up.
3.2.4 Case 30 (Fig. 3.21)

Case description
Prior history and clinical question: This case, fully elucidated, was kindly furnished to the author for teaching purposes by Dr. M. Venator, a radiologist practicing in Cologne. A 23-year-old woman had been experiencing severe right paravertebral pain in the lower thoracic spine for approximately 4 weeks. She could not recall acute antecedent trauma. The patient works as a geriatric nurse. This requires considerable lifting, and occasionally she must catch a falling patient without warning. Her family doctor found elevated serum inflammatory markers. Initial MRI demonstrated a conspicuous but initially indeterminate mass in the right T9–T10 region, which was correctly investigated further by CT on the same day. Finally a CT-guided percutaneous biopsy was performed (Fig. 3.21 j) for a presumptive diagnosis of myositis ossificans. The pathologist confirmed myositis ossificans of the thoracic spine.
Radiologic Findings
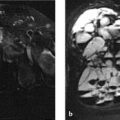
The initial MR images (Fig. 3.21 a–c) show a hyperintense right inter- and paravertebral process at the T9–T10 level that extends into the corresponding intercostal space and enhances markedly after contrast administration. The T1w images show possible evidence of an underlying osseous process, but this is not definite. The CT images in Fig. 3.21 d–i demonstrate a mass with a calcified rim (see arrows in Fig. 3.21 d, f) located in the angle between the pedicle and transverse process of T9 and T10 on the right side. No ossification is visible at the center of the lesion. CT images 1 year later (Fig. 3.21 k–m) show the now-solid ossified mass with a well-defined cortex and central medullary cavity. It has fused to the upper border of the right pedicle of T10, like an exostosis.
Location
The epicenter of the mass with the calcified or ossified rim is located in the soft tissues between two vertebral pedicles.
Pathoanatomic Background of the Findings
The MRI and CT findings can be interpreted as showing a proliferating and ossifying connective-tissue mass, but a bone-forming tumor with perifocal edema should also be considered.
Assignment to a Possible Basic Entity
Inflammation?
The good perfusion of the mass on contrast-enhanced MRI could be interpreted as inflammation-related, but not the ringlike ossification. If the term “myositis ossificans” is taken literally, since the process does appear inflammatory by its clinical and MRI features, then we are justified in classifying the mass as inflammatory.
Tumor?
Bone-forming tumors of soft tissues are extremely rare. Possibilities in the present case might include an osteoblastoma or osteosarcoma of the soft tissues.
Synopsis and Discussion
The classification of the process between the T9 and T10 pedicles on the right side as “myositis ossificans” is justifiable on radiologic grounds: an ossification develops at the center of a traumatized soft-tissue area with reactive connective-tissue proliferation. Although the patient could not recall a specific injury, her occupation as a geriatric nurse is consistent with a sudden straining movement leading to hemorrhage. On the other hand, gross trauma need not always be present as a precipitating cause of myositis ossificans; it is sufficient to have several muscular and collagen fiber tears occurring in response to minor trauma.
It is beyond our scope to explore the complex histomorphologic background of myositis ossificans, but we can briefly review its pathogenesis18 (see also Case 156):
The proliferation of spindle cells with mitotic activity occurs during the first week after soft-tissue trauma.
At 7 to 10 days, primitive osteoid is formed by fibroblasts, starting at the periphery of the traumatized area.
The second week is marked by the formation of primitive cartilage and woven bone, which differentiates to trabecular bone within 2 to 5 weeks.
By approximately 6 weeks, a cellular center with large mesenchymal cells can be seen that is surrounded by osteoid and a rim of lamellar bone. This trizonal pattern is demonstrable on radiographs and CT scans by 4 to 6 weeks at the latest.
The present case fits with the above timetable of myositis ossificans. One year after the initial visualization of ringlike ossification in a pseudoinflammatory setting, the lesion has fully differentiated and matured and has fused to the upper cortex of the T10 pedicle, like an exostosis. If these images were interpreted without knowing the prior history, the initial interpretation would probably be a hyperostotic normal variant or even a mature cartilaginous exostosis (which would then be included in the sixth edition of Freyschmidt’s “Koehler/Zimmer: Borderlands of Normal and Early Pathological Findings in Skeletal Radiography”).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree