7.2 Predominantly Osteolytic Changes
7.2.1 Case 132 (Fig. 7.7)

Case description
Referring physician: pediatric surgeon.
Prior history and clinical question: A 9-year-old boy felt pain in the left groin area after playing soccer. He had previously been free of complaints. The pediatric surgeon suspected a bone cyst and asked if his diagnosis was correct.
Radiologic Findings
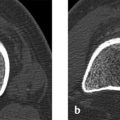
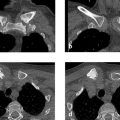
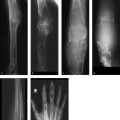
Radiographs (Fig. 7.7 a, b) demonstrate a large osteolytic area in the left femoral neck extending into the intertrochanteric region and corresponding to Lodwick grade IA. The axial projection shows that the osteolytic area is expansile with a trabeculated appearance. The T2w image demonstrates water signal intensity throughout the osteolytic area (Fig. 7.7 c), which shows peripheral enhancement after IV contrast injection (Fig. 7.7 d). The axial image in Fig. 7.7 e displays a fluid level or sedimentation.
Location
The lesion is intraosseous and occupies the entire femoral neck from the epiphyseal plate to the intertrochanteric region.
Pathoanatomic Background of the Findings
The fluid level and strictly peripheral enhancement of the lesion indicate a fluid-filled cystic process. The enhancing rim could represent the wall of a unicameral bone cyst. The fluid level also indicates intracystic hemorrhage with subsequent sedimentation of corpuscular elements. The trabecular pattern visible in the axial radiograph is caused by ridgelike bony prominences which, like the concentric expansion, are typical of a unicameral bone cyst.
Assignment to a Possible Basic Entity
The lesion was assigned to a basic entity under Pathoanatomic Background of the Findings, as there is no other reasonable possibility.
Synopsis and Discussion
The osteolytic lesion in the left femoral neck and intertrochanteric region corresponds to a unicameral bone cyst. Since the patient is a child, the bone cyst is also described as “juvenile.” Below we shall review the key radiologic and clinical findings that are characteristic of a juvenile bone cyst:
Age of the patient and location: Approximately 70 to 80% of all cases are detected in the first or second decade of life. They almost exclusively involve the metadiaphyseal region of a long bone (ca. 51% in the proximal humerus, 30% in the proximal femur).
Clinical presentation: Patients with a juvenile bone cyst typically have an extremely short history. Except in cases with a spontaneous fracture or, more commonly, a fissure fracture, the patients are free of complaints.
Radiology:
Detection of fluid contents, most clearly manifested by a fluid level and by enhancement confined to a peripheral rim. The rim enhancement occurs in the cyst wall, which is pathognomonic for histologists; without it, they cannot make a confident diagnosis of juvenile bone cyst. The wall is composed of a thin membrane of dense fibrous tissue that is partially hyalinized and generally hypocellular. The cyst wall appears grossly as a fibrous membrane, 1 mm in thickness and of a grayish-white color. It is no wonder that many percutaneous biopsies are inconclusive, as it is largely a matter of chance whether the needle samples the cyst wall. In any case, percutaneous biopsies are unnecessary in most cases because a confident diagnosis can be made from radiologic findings.
The “fallen fragment” sign: A minor injury displaces a bone fragment from the thinnest site into the lesion. There it descends through the cyst fluid to the most dependent part of the lesion, often appearing radiographically as a “fallen fragment” when imaged in a tangential projection. In our case it was not visualized as this would have required either a positioned view with a clear projection of the lowest part of the cyst or a CT scan at that level. But this was unnecessary since the MRI findings were conclusive. In principle, however, a radiograph showing a more or less expansile lesion of Lodwick grade IA–IB in the metadiaphysis of a long bone with a “fallen fragment” sign provides adequate grounds for diagnosing a unicameral bone cyst. Fig. 7.8 illustrates another case of a juvenile bone cyst, located this time in the humerus and displaying two “fallen fragments” (arrows).

Fig. 7.8 “Fallen fragment” sign of a juvenile bone cyst.
Final Diagnosis
Juvenile bone cyst in the femoral neck and intertrochanteric region.
Comments
The detection of a cyst wall by contrast-enhanced MRI and/or a fallen fragment in a Lodwick grade IA–IB lesion in the metadiaphysis of a long bone is pathognomonic for a juvenile bone cyst.
7.2.2 Case 133 (Fig. 7.9)

Case description
Referral: self-referral by the patient.
Prior history and clinical question: A 40-year-old man was found incidentally to have an osteolytic lesion in the inter- and subtrochanteric region of the left femur in 1996. Radiographs were repeated 5 years later and showed practically no change. The patient asked us if there was a risk of instability and wanted to have a definitive diagnosis.
Radiologic Findings
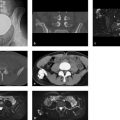
Radiographs demonstrate a Lodwick grade IB osteolytic lesion with a trabecular structure in the left intertrochanteric region (Fig. 7.9 a–c). The lesion is not expansile. The whole-body bone scan (Fig. 7.9 d) shows slightly increased uptake in the left intertrochanteric region. T1w MRI and CT scans (Fig. 7.9 e–i) show that the lesion consists of at least four components, one of which is definitely fat.
Location
The lesion is located precisely in the intertrochanteric (metadiaphyseal) region and is confined to the medullary cavity.
Pathoanatomic Background of the Findings
The aforementioned multicomponent structure makes classification of the lesion difficult. The absence of complaints and lack of change over a 5-year period suggest a benign process. This is also indicated by the substantial fatty component. Water-sensitive and contrast-enhanced MRI sequences (not pictured here) suggested that the other components consisted of connective tissue, a myxomatous component, and ossified tissue.
Assignment to a Possible Basic Entity
Normal variant or malformation?
No, at least no known precedent—unlike the lesion in Case 73, which was definitely open to that interpretation.
Trauma?
No specific history, either for a single acute trauma or for chronic repetitive trauma.
Inflammation?
No clinical manifestations, and the detection of fat does not support a diagnosis of osteomyelitis, at least an active form. The history shows no evidence of a reactive inflammatory process.
Tumor or tumorlike lesion?
Yes, see details under Synopsis and Discussion.
Synopsis and Discussion
A lesion replacing normal cancellous bone in the left intertrochanteric region was detected incidentally. It is very difficult to reconcile its four components when assigning it to a particular entity. Lesions of this kind may develop from pre-existing benign entities due to regressive processes such as circumscribed necrosis with liquefaction and later fatty infiltration (replacement by fat) or degenerative myxomatous changes. When such lesions occur in the intertrochanteric region, the pre-existing entity may well be fibrous dysplasia, since that area is a site of predilection for fibrous dysplasia in the femur. Another possible precursor lesion is benign fibrous histiocytoma, or even an intraosseous lipoma with heavy regressive changes, although this would require a dominant fatty component (see Fig. 4.42 in Case 73). A final candidate is an old bone cyst, although the femoral neck would be a more typical site of occurrence for that lesion (see Case 132).
Such lesions are relatively common incidental findings in daily practice, and the need for biopsy is a question that is often addressed. We advise against it. It will not yield a definitive histologic diagnosis, since a large specimen will contain multiple components, analogous to the imaging pattern, and the pathologist will be unable to establish a single entity. This would require a molecular pathology test (in a non–acid-decalcified specimen) to detect the GNAS-1 gene mutation that would prove fibrous dysplasia. All of the potential precursor entities listed above consist of only two, three, or at most four components, one of which may be predominant. This dominant component makes them easier to classify histologically and radiologically, as illustrated by woven bone with a ground-glass pattern in fibrous dysplasia, connective tissue in a benign fibrous histiocytoma, or fatty tissue in a lipoma.
Kransdorf et al (1999)51 and Heim-Hall and Williams (2004)52 coined the somewhat cumbersome term “liposclerosing myxofibrous tumor” in an attempt to describe the many components of fibro-osseous lesions with a predilection for the intertrochanteric region. Among the 39 cases reviewed by Kransdorf et al, four patients showed evidence of sarcomatous transformation, and all four were symptomatic. We feel that patients who are asymptomatic do not require biopsy or curettage (“leave me alone” or “don’t touch me” lesion) or even continual radiologic follow-ups, although it may be very helpful to obtain an initial bone scan showing little or no tracer uptake. We also recommend use of the well-established term “fibro-osseous lesion.” A detailed discussion of this lesion can be found in Freyschmidt et al (2010).18
As far as stability issues are concerned, the sectional images in particular show in our patient that the lesion did not cause significant bone destruction. This fact, plus the associated buttressing effect, should preclude the risk of a spontaneous fracture.
Final Diagnosis
Fibro-osseous lesion detected incidentally in the left intertrochanteric region; no need for biopsy.
Comments
If sectional imaging reveals at least three different tissue components in a lesion detected incidentally in the intertrochanteric region, the most likely diagnosis is a fibro-osseous lesion. Diagnostic confidence is further increased by a bone scan showing little or no tracer uptake in the affected region.
7.2.3 Case 134 (Fig. 7.10)

Case description
Referring physician: family doctor.
Prior history and clinical question: A 44-year-old woman had a long history of pain in both thighs, with associated equivocal radiologic changes. A biopsy of the right femur was inconclusive.
Radiologic Findings
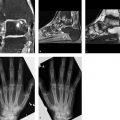
Radiographs demonstrate fine vermiform or serpiginous cortical lucencies in both femurs (only the right side is shown in Fig. 7.10 a, b). The changes were still very subtle in the 1999 radiograph (Fig. 7.10 a) and are much more pronounced by 2004 (Fig. 7.10 b). The larger defect in the proximal diaphysis is from a biopsy 6 months earlier. The T2w MR image in Fig. 7.10 c shows linear and focal hyperintensities along the area of the radiographic abnormality. MR angiography (MRA) (Fig. 7.10 d) and digital subtraction angiography (DSA) of the femoral arteries did not add useful information.
Location
The imaging abnormalities are limited to the cortical bone on both sides.
Pathoanatomic Background of the Findings
The radiographic and MRI findings are easily identified as vascular structures. They represent “vascular prints.”
Assignment to a Possible Basic Entity
Normal variant or malformation?
No, this interpretation is not supported by the progression of findings over a 5-year period.
Trauma?
No trauma history.
Inflammation?
No clinical manifestations of inflammation or a reactive inflammatory process such as psoriasis.
Tumor or tumorlike lesion?
Because we are dealing with vascular structures, only two causal entities could be considered: a vascular malformation or angiomatosis. To resolve this question, we performed direct CT venography of the lower limb (Fig. 7.10 e–i) with contrast injection into a dorsal pedal vein and a tourniquet on the upper thigh. Contrary to our expectations, this did not opacify any venous plexuses in the cavities of the cortical bone. This excluded a venous malformation but did not exclude a lymphatic malformation.
Synopsis and Discussion
Based on the radiologic findings and a re-evaluation of the histologic findings, we diagnosed the condition as cystic angiomatosis. The patient was treated with thalidomide, an inhibitor of vascular proliferation and angioneogenesis, which was followed by rapid improvement of symptoms. Unfortunately, we could not persuade the patient to consent to follow-up.
Key information on cystic angiomatosis is presented in Case 33 and Case 34. Here, we shall note again below that in dealing with vascular pathology, it is important to distinguish between vascular malformations on the one hand and vascular neoplasms on the other.
A vascular malformation may be arteriovenous with a fistula (high flow), purely venous (slow flow), lymphatic (slow flow), or a complex form. The first two types in bone can be differentiated by various current imaging techniques (MRA, DSA, CT angiography, CT venography). Lymphatic vascular malformations can be identified only histologically. With neoplastic vascular lesions, a distinction is made between malignant and benign tumors.
Hemangiomatosis is classified as a benign lesion. It is also called “angiomatosis” or, in the case of lytic lesions, “cystic hemangiomatosis.” It is defined as the occurrence of multiple hemangiomas in bone and/or soft tissues, all with the same clinical, radiologic, and histologic characteristics. While simple hemangiomas of the skin or bone generally occur in infancy and childhood and regress spontaneously with puberty, it is our experience that hemangiomas usually do not become symptomatic until adulthood. They may resolve spontaneously or may be progressive, (see Case 33). Occurrence in adulthood and the potential for progression is a feature they share with vascular malformations. This raises the question of whether at least some observed cases of hemangiomatosis are vascular malformations. This is suggested in immunohistochemical studies by Bruder et al (2009),33 who found that vascular malformations of bone had a proliferation rate of less than 1%, were GLUT1-negative, and minimally WT1-positive, which would not be consistent with neoplasms. If we consider that hemangiomas can be difficult to distinguish from vascular malformations by conventional histologic methods, there is good reason to doubt the histologic diagnoses of some benign vascular lesions, and it would be better to base the final diagnosis on radiologic criteria including the presence or absence of flow characteristics.
In our case, we exhausted all available radiologic options (see under Radiologic Findings), placing special emphasis on direct CT venography, which helped to exclude a venous intraosseous vascular malformation. In the case of a malformation, we would have performed a vascular occlusion by interventional radiology.
Final Diagnosis
Cystic angiomatosis in both femurs.
Comments
The description of serpiginous or vermiform lucencies forming vascular prints in a bone should immediately suggest a hemangioma or vascular malformation, which can often be differentiated by radiologic means.
7.2.4 Case 135 (Fig. 7.11)

Case description
Referral: self-referral by the patient.
Prior history and clinical question: A 43-year-old man underwent MRI for the investigation of suspected meniscal symptoms, and a lesion was detected incidentally in the distal metaphysis of the right femur. When neither a diagnosis nor prognosis was forthcoming, the patient asked for our opinion.
Radiologic Findings
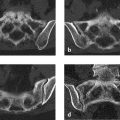
The radiographs in Fig. 7.11 a, b demonstrate an osteolytic lesion in the lateral side of the distal femoral metaphysis, corresponding to Lodwick grade IA. A latticework of sclerotic trabeculations is visible within the lesion. In the T1w images (Fig. 7.11 c, d), the lesion has high signal intensity and is isointense to the infrapatellar fat pad. The signal intensity in the fat-suppressed image (Fig. 7.11 e) approximately equals that of subcutaneous fat. To obtain more information on the osseous structures, we ordered CT scans (Fig. 7.11 f, g) which not only confirmed the fatty content of the lesion but also supplied two possible explanation for the lattice of sclerotic trabeculations, viz. (1) irregular ossifications, probably woven bone, arising from the tumor margin, or (2) residual cancellous trabeculae that were not resorbed by the tumor.
Location
The lesion is entirely intraosseous, located within the distal femoral metaphysis.
Pathoanatomic Background of the Findings
The lesion is composed mainly of fat and bony elements, which may be interpreted as residues of incomplete trabecular resorption by the tumor, may be metaplastic in nature, or may represent a buttressing process.
Assignment to a Possible Basic Entity
Normal variant or malformation?
By its nature, an incidental finding usually raises the possibility of a normal variant. In cases of disuse osteoporosis due to prolonged inactivity, fat may accumulate in the enlarged medullary cavity, but this should occur in the epiphysis and not in an eccentric pattern as in our case.
Trauma or inflammation?
There is no clinical evidence for either of these basic entities.
Tumor or tumorlike lesion?
Yes, based on the structure of the lesion, which has replaced normal osseous structures. The preponderance of fat suggests a lipoma (see Synopsis and Discussion).
Synopsis and Discussion
Because fat is predominant in the Lodwick grade IA lesion, it should be interpreted as an intraosseous lipoma. According to the WHO definition,15 “lipoma of bone is a benign neoplasm of adipocytes that arises within the medullary cavity, cortex, or on the surface of bone.” Many authors question whether lipomas are true neoplasms, suggesting that they more closely resemble a hamartoma. They may also result from the fatty transformation of old fibrous dysplasia, old bone cysts that have healed with fatty replacement, or even giant cell tumors that have resolved by fatty transformation (see also Case 133). A diagnosis of lipoma should always be made cautiously when dealing with large intraosseous collections of fatty tissue in regions that physiologically have a high fat content, such as the femoral neck (see Case 73) or the body of the calcaneus. Larger lipomas, in particular, may undergo regressive changes over time, the most common of which is central fat infarction. If the necrotic area calcifies (necrotic fat is a calcium scavenger) or undergoes dystrophic ossification, a radiograph or CT scan will demonstrate a calcific nidus (e.g., in the calcaneus). Most intraosseous lipomas that we diagnose do not require histologic evaluation if their principal component is fat, since CT or MRI can identify fat with very high confidence. Because lipomas generally grow very slowly and have already been present for years when detected (Lodwick grade IA!), they do not pose a risk of spontaneous fracture.
For comparison, Fig. 7.12 shows the case of a 14-year-old girl with a clinically symptomatic (painful) aneurysmal bone cyst in the medial portion of the distal femoral metaphysis. It bears a superficial resemblance to lipoma. The osteolytic lesion is classified as Lodwick grade IB (Fig. 7.12 a), indicating a faster growth rate than lipoma. While lipoma stimulates a marked buttressing effect with cortical thickening over the lesion, the cortex over the aneurysmal bone cyst is very thin and “bulging.” An aneurysmal bone cyst cannot be diagnosed from radiographs alone, so MRI was added to detect a dominant fluid level (Fig. 7.12 c) and marked enhancement of the stroma (Fig. 7.12 b), consisting of ridges or septa (see Case 45). This is pathognomonic for an aneurysmal bone cyst.

Final Diagnosis
Intraosseous lipoma detected incidentally in the medial portion of the distal femoral metaphysis.
Comments
Incidentally detected tumors composed mainly of fat according to CT or MRI measurements should be classified as lipomas and left alone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree