Abstract
Gliosarcoma is a rare and aggressive variant of glioblastoma, characterized by a biphasic histological pattern consisting of both glial and mesenchymal components. This case report describes the clinical presentation, radiological findings, surgical management, and histopathological analysis of gliosarcoma in a 30-year-old female. The patient presented with a 10-day history of right-sided headache and recurrent vomiting. Neurological examination was unremarkable, and vital signs were stable. Magnetic resonance imaging (MRI) revealed a heterogeneously enhancing mass lesion involving the right parietal region and the splenium of the corpus callosum, crossing the midline and causing significant ventricular effacement. Imaging features included heterogeneously hypointense signals on T1-weighted imaging, hyperintense signals on T2/FLAIR, areas of blooming on susceptibility-weighted imaging, and restricted diffusion on diffusion-weighted imaging, suggestive of a high-grade glial tumor. The patient underwent surgical resection, and histopathological examination confirmed gliosarcoma. The tumor exhibited a biphasic pattern comprising glial and sarcomatous elements. This case emphasizes the diagnostic challenges associated with gliosarcoma, where radiological features often mimic glioblastoma, necessitating histopathological confirmation. Gliosarcoma’s aggressive nature poses significant therapeutic challenges, with treatment strategies involving surgical resection followed by adjuvant radiotherapy and chemotherapy. This report highlights the importance of integrating clinical, radiological, and histopathological findings to achieve an accurate diagnosis and optimize treatment outcomes. It underscores the need for early recognition and a multidisciplinary approach to managing rare central nervous system tumors like gliosarcoma. Further research into advanced therapeutic strategies is warranted to improve the prognosis for such patients.
Background
Gliosarcoma, a rare and aggressive primary brain tumor, is classified as a World Health Organization (WHO) grade IV glioma. Representing approximately 2% of all glioblastomas, gliosarcoma is distinguished by its unique biphasic histological pattern, comprising both glial and sarcomatous components [ , ]. This tumor typically affects adults, with a peak incidence in the fifth to sixth decade of life, and shows a slight male predominance [ ]. Despite its low incidence, gliosarcoma’s aggressive behavior and poor prognosis make it a significant clinical entity requiring detailed study. Most tumors are located in the supratentorial region, with the frontal and temporal lobes being the most common sites, although rarer extracranial metastases have been reported [ , ]. Clinically, patients with gliosarcoma often present with symptoms indicative of increased intracranial pressure, such as headache, nausea, and vomiting, accompanied by focal neurological deficits, depending on the lesion’s location [ ]. The presentation can mimic other high-grade gliomas, underscoring the need for accurate diagnostic evaluation.
Radiological imaging, especially magnetic resonance imaging (MRI), is integral to the diagnostic process. Gliosarcoma frequently presents as a heterogeneously enhancing mass with areas of necrosis and significant peritumoral edema. Advanced imaging techniques such as diffusion-weighted imaging (DWI), which detects high cellularity, and susceptibility-weighted imaging (SWI), which identifies hemorrhagic components, add diagnostic value [ ]. Despite these advancements, differentiating gliosarcoma from glioblastoma solely based on imaging remains challenging, making histopathological analysis indispensable [ ].
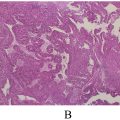
Histologically, gliosarcoma exhibits a biphasic architecture. The glial component typically resembles glioblastoma, with features such as cellular pleomorphism, necrosis, and microvascular proliferation. The sarcomatous component, characterized by spindle cells and mesenchymal differentiation, differentiates gliosarcoma from glioblastoma [ ]. This dual morphology suggests a common progenitor cell capable of divergent differentiation, further supported by molecular studies showing shared genetic mutations between the 2 components [ , ].
Treatment approaches for gliosarcoma are modeled after those for glioblastoma, including maximal safe surgical resection followed by radiotherapy and temozolomide-based chemotherapy. Despite these interventions, gliosarcoma’s prognosis remains dismal, with median survival ranging from 6 to 14 months, slightly lower than glioblastoma due to its higher rate of recurrence and resistance to therapy [ , ]. The poor outcomes highlight the need for novel therapeutic strategies, such as immunotherapy and targeted molecular therapies, which are currently under investigation [ , ].
Case presentation
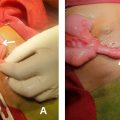
A 30-year-old female presented to the emergency department with a 10-day history of persistent right-sided headache and recurrent episodes of vomiting. She denied any associated symptoms, including trauma, neurological deficits, fever, or any prior history of chronic illnesses such as hypertension or diabetes mellitus. Her personal and family medical histories were unremarkable. Upon clinical examination, the patient was alert and oriented to time, place, and person, and her vital signs were within normal limits. The neurological assessment showed no focal abnormalities.
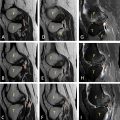
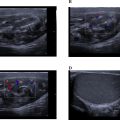
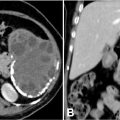
Given the nature and persistence of her symptoms, an MRI of the brain was conducted. The imaging revealed a heterogeneously enhancing mass lesion located in the right parietal region and the splenium of the corpus callosum. The lesion was noted to cross the midline. It caused significant effacement of the occipital horns of the bilateral lateral ventricles and the third ventricle, leading to features consistent with obstructive hydrocephalus. On MRI, the lesion appeared heterogeneously hypointense on T1-weighted imaging (T1WI) and heterogeneously hyperintense on T2/FLAIR sequences, indicating the presence of a mixed solid and edematous component Figures 1-2 . Susceptibility-weighted imaging (SWI) demonstrated areas of blooming, suggestive of intralesional hemorrhage, while diffusion-weighted imaging (DWI) revealed areas of restricted diffusion, indicative of high cellular density and tumor aggressiveness Figures 3-4 . These findings raised a strong suspicion of glioblastoma multiforme, a highly aggressive primary brain tumor.





Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree