Balloon-Occluded Retrograde Transvenous Obliteration
Nicholas J. Hendricks
Ziv J. Haskal
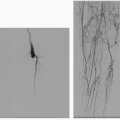
Gastric variceal bleeding is a life-threatening complication of portal hypertension. Balloon-occluded retrograde transvenous obliteration (BRTO) is a procedure by which upstream occlusion of a pathologic portosystemic shunt is achieved using sclerosants and/or embolic solutions. Retrograde transvenous obliteration of gastric varices using sclerosants such as ethanolamine has been used in Asia for over
20 years (1). In recent years, it has become widely propagated in the West, and technique, device, and agents used have evolved. Variations of this procedure include plug- and coil-assisted techniques using gelfoam instead of sclerosant agents, which have shown to be quite effective (2).
20 years (1). In recent years, it has become widely propagated in the West, and technique, device, and agents used have evolved. Variations of this procedure include plug- and coil-assisted techniques using gelfoam instead of sclerosant agents, which have shown to be quite effective (2).
Indications
1. Secondary prevention of recurrent gastric variceal hemorrhage. In most cases, BRTO (and variants) are not performed during acute variceal hemorrhage, although procedural evolution may allow its increasing use in this setting.
2. Primary prophylaxis of gastric variceal hemorrhage. In contrast to Western hemisphere, this is a common indication in Asia. Comparing published results of respective series must take this into account.
3. Treatment of excess portosystemic encephalopathy (shunting-type) aggravated portal hypertension or secondary development of ascites or increased esophageal varices. Improved liver function can occur after such shunt obliteration (3).
4. Bleeding ectopic varices (e.g., duodenal). Anatomically, all target varices (be they gastric or ectopic) must have a catheter-accessible efferent drainage (e.g., gastrorenal or gastrocaval shunt).
Contraindications
Absolute
1. Uncorrected coagulopathy
2. Patient is too unstable to tolerate time required for procedure. These patients may be more suitable for transjugular intrahepatic portosystemic shunt (TIPS).
Relative
1. Refractory ascites
2. Severe liver dysfunction
3. Associated high-risk esophageal varices
Preprocedure Preparation
1. Informed consent
2. Preprocedure imaging with either triple phase contrast computed tomography (CT) or magnetic resonance angiography (MRA)/magnetic resonance venography (MRV) to evaluate the portal vein and the extent of portosystemic shunts. CT may be advantageous due to its accessibility because it still allows multiplanar reconstructions. It is important to evaluate the size of the gastrorenal shunt for suitability of the procedure and device selection. It is also important to understand the portal inflow to the shunt. This can be difficult because some of these shunts can have multiple small inflow veins. Imaging can also be used to identify collateral veins that may require embolization prior to instillation of sclerosants (e.g., phrenic or pericardiophrenic veins).
3. Laboratory evaluation to include basic metabolic and electrolyte panels, complete blood counts, coagulation parameters, and liver function tests. Platelet counts above 50,000 are adequate to perform this procedure.
4. Hemodynamic monitoring with electrocardiography, blood pressure, and oxygen saturation
5. Aggressive resuscitation should be implemented in acutely bleeding patients with hemodynamic instability. This may require packed red blood cells, fresh frozen plasma, platelets, cryoglobulin, pressor support, and intravenous (IV) fluids.
6. Conscious sedation using IV fentanyl and midazolam is suitable for most patients.
7. Accumulate the required components:
a. Embolic versus sclerosant agent
(1) Sodium tetradecyl sulfate (Sotradecol, AngioDynamics, Queensbury, NY)
(2) Ethanolamine oleate (Oldamin, Grelan Pharmaceutical, Tokyo, Japan) Haptoglobin is often required as part of its use.
(3) Polidocanol foam (Polidocasklerol, ZERIA Pharmaceutical, Tokyo, Japan)
(4) n-Butyl cyanoacrylate
(5) Gelfoam (Pharmacia & Upjohn, New York, NY) is used during plugassisted retrograde transvenous obliteration (PARTO) procedures.
b. Balloon-occlusion catheters (others may be equally suitable)
(1) Coda (Cook Medical, Bloomington, IN)
(2) Python occlusion balloon (Applied Medical, Rancho Santa Margarita, CA)
(3) Equalizer (Boston Scientific Corporation, Natick, MA)
c. Plugs (needed for PARTO technique)
(1) Amplatzer plug (St. Jude Medical, St. Paul, MN)
d. Coils (for coil-assisted techniques)
(1) Pushable versus detachable, metallic, or polymer embolization coils
Procedure
Balloon-Occluded Retrograde Transvenous Obliteration
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree