Basic Physics of Single Photon Emission Computed Tomography Physics
Kusai S. Aziz
This chapter does not deal with the details of nuclear physics, but rather emphasizes basic must-know concepts of nuclear physics that are needed in the practice of nuclear cardiology. Many of these concepts tend to be the subject of board exam questions because of their importance.
Definitions and Basic Principles
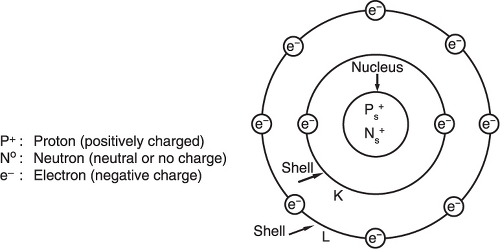
Basic atomic structure: each atom is composed of a nucleus that contains protons and neutrons, and shells that contain electrons (Figure 1.1).
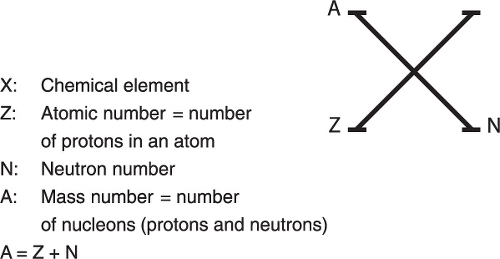
Elements are expressed in a standard fashion to reflect nuclear characteristics (Figure 1.2).
The unit of energy is electron volts (eV), defined as the energy or acceleration of an electron obtained by applying electric potential of one volt.
Different electromagnetic forces have different levels of energy that increase in an incremental manner: radio waves < TV waves < radar waves < infrared light < visible light < ultraviolet rays < X rays < gamma rays.
The frequency and photon energy of any electromagnetic force is inversely related to its wavelength.
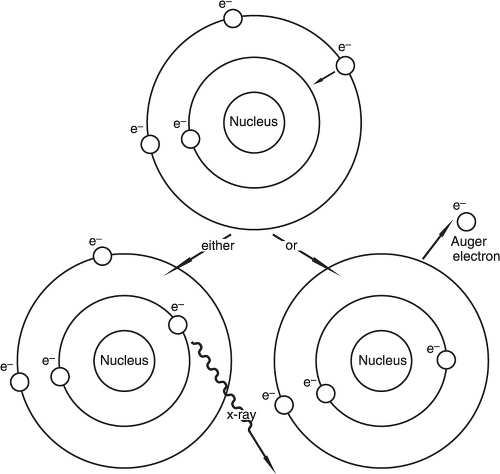
When an electron moves from an outer shell (higher energy) to an inner shell (lower energy), the excess energy is emitted as a characteristic X-ray or as an Auger electron, which is another electron emitted from an outer shell (Figure 1.3). The probability of X-ray emission versus Auger electron is directly related to the atomic number.
Energy and mass are interchangeable: Energy = mass × c (constant, velocity of light).
Isotopes have the same number of protons (atomic number, Z); isotones have the same neutron number, N; and isobars have the same mass number, A. Isomers are nuclides of the same element with one of them being in a metastable (unstable) state, e.g., Tc-99m (99mTc) and 99Tc are isomers. The metastable isomer tends to decay to the stable form by emitting radiation.
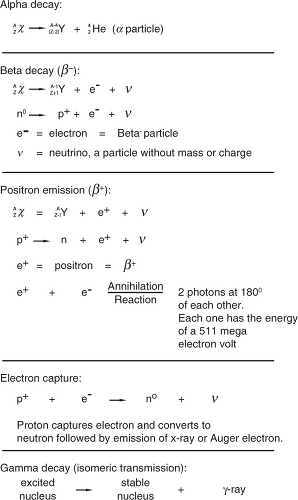
Radioactive decay: a spontaneous nuclear process in which an unstable nucleus transforms to more stable forms by emitting particles or photons. The original nucleus is called “parent” and the new nucleus is called “daughter,” which itself can be a parent and decay further to another “daughter” nucleus. There are different types of decay, as illustrated in Figure 1.4.
Units of radioactivity:
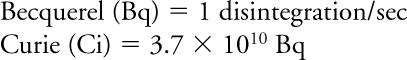
The Bq is an SI unit; most medical applications still use the old system (Ci).
Physical half-life: the time required for a specific amount of radionuclide or activity to decay to half its original amount. Biological half-life is the time required for the chemical portion of radionuclide to decrease to half its original amount in biological systems, which includes metabolism and excretion. Effective half-life is the time required for the radioactive material to decrease to half its original quantity and this depends on both physical and biological factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree