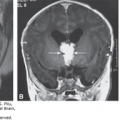
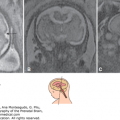
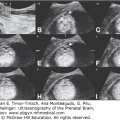
Prenatal diagnosis should be instituted accurately and early enough to achieve an excellent level of care of the obstetric patient. Ultrasonography, with its increasingly better resolution and image quality, is making it easier for health care providers to make appropriate management decisions. Assessing whether a structure is normal or abnormal may not always be feasible, but if doubt exists regarding its normalcy, it must be carefully and diligently pursued. In addition, several measurements, such as the biparietal diameter (BPD) and the head circumference (HC), can be used to determine the gestational age of a fetus, especially during the first half of pregnancy. Correct estimation of gestational age is of paramount importance because adequate management of both low- and high-risk obstetric populations relies heavily on knowing the precise gestational age.
The tables in this chapter have been compiled from the literature for the sole purpose of serving as an easy reference against which measurements can be compared. An attempt has been made to include as many tables of different parameters as possible. These tables, assembled in a single chapter, will allow the sonographer or sonologist to more easily make the differentiation between normal and abnormal measurements, without having to search different textbooks and articles for a particular measurement.
This chapter is divided into four main sections. The first, crown-rump length (CRL), although not a brain or head measurement, is included to provide a means of predicting embryonic or fetal age. This parameter is of obvious importance and eliminates the need to turn to another source.
The second section, dealing with head measurements, can be used not only to date the pregnancy but also to aid in the diagnosis of microcephaly and alterations in fetal head shape. This section also includes measurements of orbital diameters, which can be of help in the diagnosis of eye pathology.
The third section provides a variety of tables concerning the different portions of the fetal ventricular system, mainly for the purpose of making early diagnosis of ventriculomegaly possible. Congenital hydrocephaly is one of the most frequently described anomalies, with an incidence of 0.3 to 1.5 per 1000 births. The importance of in utero detection of this anomaly cannot be overemphasized. This section also includes tables that we have generated using the transvaginal-transfontanelle approach to the fetal brain using 5 to 7.5 MHz transvaginal probes. These tables enhance and complement the widely accepted transabdominally generated tables, thereby advancing the field of fetal neurosonography and giving new meaning to the term early diagnosis.
The fourth and final section includes measurements of other intracranial structures, such as the thalami, basal nuclei, cerebellum, and cerebellomedullary cistern. These can assist the sonographer or sonologist in the diagnosis of pathologies such as Dandy-Walker and Arnold-Chiari malformations.
This chapter attempts to provide the reader with a unique reference guide to fetal brain measurements.
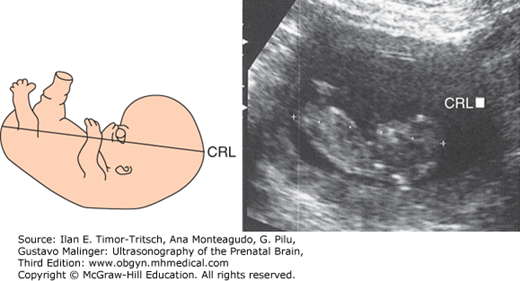
The CRL represents the longest measurable length of the embryo or fetus, excluding the inferior limbs or the yolk sac.1,2
In a longitudinal scan, the CRL is measured from a point immediately over the middle of the midbrain (crown) to the embryonic or fetal rump (Figure 3–1). O’Rahilly and Müller1 suggested that from 28 to 44 postovulatory days (6.0 to 8.5 postmenstrual weeks,* or Carnegie stages 13 to 18), the maximum longitudinal length of the embryo is not truly represented by the CRL, but by what they called the greatest length. This is explained by the fact that during these stages, the normal flexion of the embryonic head locates the middle of the midbrain below the highest point of the head. As pregnancy advances, the head extends, making the middle of the midbrain match the highest point of the head and thus becoming a true “crown” reference point (see Chapters 1 and 2).
*Fetal and embryonic age is described here in reference to postmenstrual weeks. Postovulatory age is approximately 2 weeks less than postmenstrual age.
Although the CRL is obviously not a measurement of the brain, it will be included in this chapter as a reference for the assessment of estimated age, a parameter of utmost importance when evaluating the embryo and the fetus, and one against which most biometry tables are plotted. The validity of this parameter for determination of age in the first trimester has been widely reported in the literature.2,3,4 The average of three measurements should be used to diminish random errors in the technique. The variability in predicting postmenstrual age with the CRL changes over time, with a reported range of error of ± 8% of the estimate.2 Possible factors contributing to this range are (1) normal biologic variation in fetal size, (2) variation in the times of ovulation and fertilization, and (3) errors attributed to the measurement technique.3 Nevertheless, the CRL remains the gold standard measurement for dating pregnancy in the first trimester, with the exception of in vitro fertilization cases (Tables 3–1 and 3–2).
| Postmenstrual Gestational Age (weeks + days) | CRL (mm) | Postmenstrual Gestational Age (weeks + days) | CRL (mm) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 6 + 2 | 6.7 | 2.9 | 10 + 2 | 35.5 | 6.9 |
| 6 + 3 | 7.4 | 3.1 | 10 + 3 | 36.9 | 7.0 |
| 6 + 4 | 8.0 | 3.2 | 10 + 4 | 38.4 | 7.2 |
| 6 + 5 | 8.7 | 3.4 | 10 + 5 | 39.9 | 7.3 |
| 6 + 6 | 9.5 | 3.5 | 10 + 6 | 41.4 | 7.4 |
| 7 + 0 | 10.2 | 3.7 | 11 + 0 | 43.0 | 7.6 |
| 7 + 1 | 11.0 | 3.8 | 11 + 1 | 44.6 | 7.7 |
| 7 + 2 | 11.8 | 3.9 | 11 + 2 | 46.2 | 7.9 |
| 7 + 3 | 12.6 | 4.1 | 11 + 3 | 47.8 | 8.0 |
| 7 + 4 | 13.5 | 4.2 | 11 + 4 | 49.5 | 8.1 |
| 7 + 5 | 14.4 | 4.4 | 11 + 5 | 51.2 | 8.3 |
| 7 + 6 | 15.3 | 4.5 | 11 + 6 | 52.9 | 8.4 |
| 8 + 0 | 16.3 | 4.6 | 12 + 0 | 54.7 | 8.6 |
| 8 + 1 | 17.3 | 4.8 | 12 + 1 | 56.5 | 8.7 |
| 8 + 2 | 18.3 | 4.9 | 12 + 2 | 58.3 | 8.8 |
| 8 + 3 | 19.3 | 5.1 | 12 + 3 | 60.1 | 9.0 |
| 8 + 4 | 20.4 | 5.2 | 12 + 4 | 62.0 | 9.1 |
| 8 + 5 | 21.5 | 5.3 | 12 + 5 | 63.9 | 9.3 |
| 8 + 6 | 22.6 | 5.5 | 12 + 6 | 65.9 | 9.4 |
| 9 + 0 | 23.8 | 5.6 | 13 + 0 | 67.8 | 9.5 |
| 9 + 1 | 25.0 | 5.8 | 13 + 1 | 69.8 | 9.7 |
| 9 + 2 | 26.2 | 5.9 | 13 + 2 | 71.8 | 9.8 |
| 9 + 3 | 27.4 | 6.0 | 13 + 3 | 73.9 | 10.0 |
| 9 + 4 | 28.7 | 6.2 | 13 + 4 | 76.0 | 10.1 |
| 9 + 5 | 30.0 | 6.3 | 13 + 5 | 78.1 | 10.2 |
| 9 + 6 | 31.3 | 6.5 | 13 + 6 | 80.2 | 10.4 |
| 10 + 0 | 32.7 | 6.6 | 14 + 0 | 82.4 | 10.5 |
| 10 + 1 | 34.0 | 6.7 | |||
| CRL (cm) | MA (weeks) | CRL (cm) | MA (weeks) | CRL (cm) | MA (weeks) |
|---|---|---|---|---|---|
| 0.2 | 5.7 | 4.2 | 11.1 | 8.2 | 14.2 |
| 0.3 | 5.9 | 4.3 | 11.2 | 8.3 | 14.2 |
| 0.4 | 6.1 | 4.4 | 11.2 | 8.4 | 14.3 |
| 0.5 | 6.2 | 4.5 | 11.3 | 8.5 | 14.4 |
| 0.6 | 6.4 | 4.6 | 11.4 | 8.6 | 14.5 |
| 0.7 | 6.6 | 4.7 | 11.5 | 8.7 | 14.6 |
| 0.8 | 6.7 | 4.8 | 11.6 | 8.8 | 14.7 |
| 0.9 | 6.9 | 4.9 | 11.7 | 8.9 | 14.8 |
| 1.0 | 7.2 | 5.0 | 11.7 | 9.0 | 14.9 |
| 1.1 | 7.2 | 5.1 | 11.8 | 9.1 | 15.0 |
| 1.2 | 7.4 | 5.2 | 11.9 | 9.2 | 15.1 |
| 1.3 | 7.5 | 5.3 | 12.0 | 9.3 | 15.2 |
| 1.4 | 7.7 | 5.4 | 12.0 | 9.4 | 15.3 |
| 1.5 | 7.9 | 5.5 | 12.1 | 9.5 | 15.3 |
| 1.6 | 8.0 | 5.6 | 12.2 | 9.6 | 15.4 |
| 1.7 | 8.1 | 5.7 | 12.3 | 9.7 | 15.5 |
| 1.8 | 8.3 | 5.8 | 12.3 | 9.8 | 15.6 |
| 1.9 | 8.4 | 5.9 | 12.4 | 9.9 | 15.7 |
| 2.0 | 8.6 | 6.0 | 12.5 | 10.0 | 15.9 |
| 2.1 | 8.7 | 6.1 | 12.6 | 10.1 | 16.0 |
| 2.2 | 8.9 | 6.2 | 12.6 | 10.2 | 16.1 |
| 2.3 | 9.0 | 6.3 | 12.7 | 10.3 | 16.2 |
| 2.4 | 9.1 | 6.4 | 12.8 | 10.4 | 16.3 |
| 2.5 | 9.2 | 6.5 | 12.8 | 10.5 | 16.4 |
| 2.6 | 9.4 | 6.6 | 12.9 | 10.6 | 16.5 |
| 2.7 | 9.5 | 6.7 | 13.0 | 10.7 | 16.6 |
| 2.8 | 9.6 | 6.8 | 13.1 | 10.8 | 16.7 |
| 2.9 | 9.7 | 6.9 | 13.1 | 10.9 | 16.8 |
| 3.0 | 9.9 | 7.0 | 13.2 | 11.0 | 16.9 |
| 3.1 | 10.0 | 7.1 | 13.3 | 11.1 | 17.0 |
| 3.2 | 10.1 | 7.2 | 13.4 | 11.2 | 17.1 |
| 3.3 | 10.2 | 7.3 | 13.4 | 11.3 | 17.2 |
| 3.4 | 10.3 | 7.4 | 13.5 | 11.4 | 17.3 |
| 3.5 | 10.4 | 7.5 | 13.6 | 11.5 | 17.4 |
| 3.6 | 10.5 | 7.6 | 13.7 | 11.6 | 17.5 |
| 3.7 | 10.6 | 7.7 | 13.8 | 11.7 | 17.6 |
| 3.8 | 10.7 | 7.8 | 13.8 | 11.8 | 17.7 |
| 3.9 | 10.8 | 7.9 | 13.9 | 11.9 | 17.8 |
| 4.0 | 10.9 | 8.0 | 14.0 | 12.0 | 17.9 |
| 4.1 | 11.0 | 8.1 | 14.1 | 12.1 | 18.0 |
Blaas and colleagues5 described the use of three-dimensional (3D) transvaginal sonography to outline in detail the outer contours of embryos at 7 to 10 weeks of gestation. Using this technique, they were able to describe the contours of the brain cavities, as well as calculate volumes that corresponded well with the descriptions from classic human embryology.
The BPD represents the widest transverse dimension of the fetal head.
The fetal head should be imaged in a horizontal (axial) plane (Figure 3–2). The transducer should be tilted to match the angle of inclination of the head in the vertical axis of the fetus so that a horizontal section can be obtained. This is recognized by the appearance of the midline echo and the widest fetal head diameter at right angles with it. The transducer should then be rotated (following the position of the fetal spine) until the head appears as an ovoid and a small anechoic area is detected in the midline, one-third of the distance from the sinciput.6 According to Hadlock and colleagues,7 this plane represents a section along the suboccipital-bregmatic axis, angled ~40°, to the canthomeatal line. Intracranial landmarks that should be recognized in an anterior-to-posterior fashion are (see Figure 3–2 for abbreviations) the falx cerebri (F); the cavum septi pellucidi (CSP), which corresponds to the midline anechoic area described by Campbell and Thoms;6 the cerebral peduncles (P); and again, the falx cerebri in the midline. Laterally, one should be able to recognize, depending on the age of the fetus, the anterior horns (AH) of the lateral ventricles, the hypoechoic thalami (T), and the choroid plexus (CP) in the atrium (A) of each lateral ventricle. Halfway between the thalami and the calvarium, a linear echo corresponding to the insula (I) can be seen, with the pulsating middle cerebral artery within. Once the correct plane has been identified, the calipers should be placed at the outer surface of the skull table nearest the transducer and at the inner margin of the opposite skull table, with gain settings adjusted so that the width of the skull table nearer the transducer is 3 to 5 mm.
The BPD was one of the first sonographic parameters used to estimate fetal age.8 It has been shown to be a reliable predictor of menstrual age in the first half of pregnancy, being the most accurate between 12 and 18 (± 1.2) weeks. As pregnancy progresses, the BPD loses its power to correctly predict gestational age, with a margin of error of ± 3.2 weeks at 36 to 42 weeks (Tables 3–3 and 3–4).9
| Menstrual Age (weeks) | Biparietal Diameter (cm) | Menstrual Age (weeks) | Biparietal Diameter (cm) |
|---|---|---|---|
| 12.0 | 1.7 | 26.0 | 6.5 |
| 12.5 | 1.9 | 26.5 | 6.7 |
| 13.0 | 2.1 | 27.0 | 6.8 |
| 13.5 | 2.3 | 27.5 | 6.9 |
| 14.0 | 2.5 | 28.0 | 7.1 |
| 14.5 | 2.7 | 28.5 | 7.2 |
| 15.0 | 2.9 | 29.0 | 7.3 |
| 29.5 | 7.5 | ||
| 15.5 | 3.1 | 30.0 | 7.6 |
| 16.0 | 3.2 | 30.5 | 7.7 |
| 16.5 | 3.4 | 31.0 | 7.8 |
| 17.0 | 3.6 | 31.5 | 7.9 |
| 17.5 | 3.8 | 32.0 | 8.1 |
| 18.0 | 3.9 | 32.5 | 8.2 |
| 18.5 | 4.1 | 33.0 | 8.3 |
| 19.0 | 4.3 | 33.5 | 8.4 |
| 19.5 | 4.5 | 34.0 | 8.5 |
| 20.0 | 4.6 | 34.5 | 8.6 |
| 20.5 | 4.8 | 35.0 | 8.7 |
| 21.0 | 5.0 | 35.5 | 8.8 |
| 21.5 | 5.1 | 36.0 | 8.9 |
| 22.0 | 5.3 | 36.5 | 8.9 |
| 22.5 | 5.5 | 37.0 | 9.0 |
| 23.0 | 5.6 | 37.5 | 9.1 |
| 23.5 | 5.8 | 38.0 | 9.2 |
| 24.0 | 5.9 | 38.5 | 9.2 |
| 24.5 | 6.1 | 39.0 | 9.3 |
| 25.0 | 6.2 | 39.5 | 9.4 |
| 25.5 | 6.4 | 40.0 | 9.4 |
| Gestational Age (weeks + days) | |||
|---|---|---|---|
| Biparietal Diameter (mm) | 5th Percentile | 50th Percentile | 95th Percentile |
| 10 | 7 + 0 | 10 + 1 | 13 + 1 |
| 11 | 7 + 2 | 10 + 2 | 13 + 3 |
| 12 | 7 + 3 | 10 + 4 | 13 + 4 |
| 13 | 7 + 5 | 10 + 5 | 13 + 5 |
| 14 | 7 + 6 | 10 + 6 | 14 + 0 |
| 15 | 8 + 1 | 11 + 1 | 14 + 1 |
| 16 | 8 + 2 | 11 + 2 | 14 + 3 |
| 17 | 8 + 4 | 11 + 4 | 14 + 4 |
| 18 | 8 + 5 | 11 + 5 | 14 + 6 |
| 19 | 9 + 0 | 12 + 0 | 15 + 0 |
| 20 | 9 + 1 | 12 + 2 | 15 + 2 |
| 21 | 9 + 3 | 12 + 3 | 15 + 3 |
| 22 | 9 + 4 | 12 + 5 | 15 + 5 |
| 23 | 9 + 6 | 12 + 6 | 16 + 0 |
| 24 | 10 + 1 | 13 + 1 | 16 + 1 |
| 25 | 10 + 2 | 13 + 3 | 16 + 3 |
| 26 | 10 + 4 | 13 + 4 | 16 + 5 |
| 27 | 10 + 6 | 13 + 6 | 17 + 0 |
| 28 | 11 + 0 | 14 + 1 | 17 + 1 |
| 29 | 11 + 2 | 14 + 3 | 17 + 3 |
| 30 | 11 + 4 | 14 + 4 | 17 + 5 |
| 31 | 11 + 6 | 14 + 6 | 18 + 0 |
| 32 | 12 + 1 | 15 + 1 | 18 + 1 |
| 33 | 12 + 3 | 15 + 3 | 18 + 3 |
| 34 | 12 + 4 | 15 + 5 | 18 + 5 |
| 35 | 12 + 6 | 16 + 0 | 19 + 0 |
| 36 | 13 + 1 | 16 + 2 | 19 + 2 |
| 37 | 13 + 3 | 16 + 4 | 19 + 4 |
| 38 | 13 + 5 | 16 + 6 | 19 + 6 |
| 39 | 14 + 0 | 17 + 1 | 20 + 1 |
| 40 | 14 + 2 | 17 + 3 | 20 + 3 |
| 41 | 14 + 4 | 17 + 5 | 20 + 5 |
| 42 | 14 + 6 | 18 + 0 | 21 + 0 |
| 43 | 15 + 1 | 18 + 2 | 21 + 2 |
| 44 | 15 + 3 | 18 + 4 | 21 + 4 |
| 45 | 15 + 6 | 18 + 6 | 21 + 6 |
| 46 | 16 + 1 | 19 + 1 | 22 + 1 |
| 47 | 16 + 3 | 19 + 3 | 22 + 4 |
| 48 | 16 + 5 | 19 + 5 | 22 + 6 |
| 49 | 17 + 0 | 20 + 1 | 23 + 1 |
| 50 | 17 + 3 | 20 + 3 | 23 + 3 |
| 51 | 17 + 5 | 20 + 5 | 23 + 6 |
| 52 | 18 + 0 | 21 + 0 | 24 + 1 |
| 53 | 18 + 2 | 21 + 3 | 24 + 3 |
| 54 | 18 + 5 | 21 + 5 | 24 + 5 |
| 55 | 19 + 0 | 22 + 0 | 25 + 1 |
| 56 | 19 + 2 | 22 + 3 | 25 + 3 |
| 57 | 19 + 5 | 22 + 5 | 25 + 6 |
| 58 | 20 + 0 | 23 + 1 | 26 + 1 |
| 59 | 20 + 3 | 23 + 3 | 26 + 3 |
| 60 | 20 + 5 | 23 + 6 | 26 + 6 |
| 61 | 21 + 1 | 24 + 1 | 27 + 1 |
| 62 | 21 + 3 | 24 + 4 | 27 + 4 |
| 63 | 21 + 6 | 24 + 6 | 27 + 6 |
| 64 | 22 + 1 | 25 + 2 | 28 + 2 |
| 65 | 22 + 4 | 25 + 4 | 28 + 5 |
| 66 | 22 + 6 | 26 + 0 | 29 + 0 |
| 67 | 23 + 2 | 26 + 2 | 29 + 3 |
| 68 | 23 + 5 | 26 + 5 | 29 + 5 |
| 69 | 24 + 0 | 27 + 1 | 30 + 1 |
| 70 | 24 + 3 | 27 + 3 | 30 + 4 |
| 71 | 24 + 6 | 27 + 6 | 30 + 6 |
| 72 | 25 + 1 | 28 + 2 | 31 + 2 |
| 73 | 25 + 4 | 28 + 5 | 31 + 5 |
| 74 | 26 + 0 | 29 + 0 | 32 + 1 |
| 75 | 26 + 3 | 29 + 3 | 32 + 4 |
| 76 | 26 + 6 | 29 + 6 | 32 + 6 |
| 77 | 27 + 1 | 30 + 2 | 33 + 2 |
| 78 | 27 + 4 | 30 + 5 | 33 + 5 |
| 79 | 28 + 0 | 31 + 1 | 34 + 1 |
| 80 | 28 + 3 | 31 + 3 | 34 + 4 |
| 81 | 28 + 6 | 31 + 6 | 35 + 0 |
| 82 | 29 + 2 | 32 + 2 | 35 + 3 |
| 83 | 29 + 5 | 32 + 5 | 35 + 6 |
| 84 | 30 + 1 | 33 + 1 | 36 + 2 |
| 85 | 30 + 4 | 33 + 4 | 36 + 5 |
| 86 | 31 + 0 | 34 + 0 | 37 + 1 |
| 87 | 31 + 3 | 34 + 3 | 37 + 4 |
| 88 | 31 + 6 | 35 + 0 | 38 + 0 |
| 89 | 32 + 2 | 35 + 3 | 38 + 3 |
| 90 | 32 + 5 | 35 + 6 | 38 + 6 |
| 91 | 33 + 2 | 36 + 2 | 39 + 2 |
| 92 | 33 + 5 | 36 + 5 | 39 + 6 |
| 93 | 34 + 1 | 37 + 1 | 40 + 2 |
| 94 | 34 + 4 | 37 + 5 | 40 + 5 |
| 95 | 35 + 0 | 38 + 1 | 41 + 1 |
| 96 | 35 + 4 | 38 + 4 | 41 + 4 |
| 97 | 36 + 0 | 39 + 0 | 42 + 1 |
| 98 | 36 + 3 | 39 + 4 | 42 + 4 |
| 99 | 37 + 0 | 40 + 0 | 43 + 0 |
The observed variation in the third trimester has been related to (1) technical errors in imaging, (2) genetic variations in head size in fetuses of equal age, and (3) differences in the times of ovulation and fertilization during the menstrual period.
Sonographic measurement of the BPD may be misleading if the fetal head shape is abnormal. It has been postulated that extrinsic factors such as breech presentation and oligohydramnios can alter fetal head shape.10,11 In an attempt to identify variations in the shape of the fetal skull that might adversely affect the potential of the BPD in estimating age, Hadlock and associates12 developed the so-called cephalic index (CI). When this parameter is abnormal, other measurements, such as the HC, abdominal circumference, or femur length, should be used to predict fetal age.
The CI is the relationship between the short and long axes of the fetal skull, measured at the level of the BPD.
The widest transverse and longitudinal (occipitofrontal diameter [OFD]) dimensions of the fetal skull at the level of the BPD are measured from outer margin to outer margin. The CI can then be calculated using the following simple equation:
The clinical application of the CI lies in its property to discriminate between the normal fetal head shape and the head that is abnormal enough to alter fetal age estimation based on the BPD. Thus, in situations that may modify the fetal head shape, (eg, oligohydramnios and breech presentation13,14), other parameters, such as the HC, should be used.
Hadlock and coworkers12 suggested that the CI is constant throughout gestation, with a mean value of 78.3 and a standard deviation (SD) of 4.4. A CI > 1 SD from the mean (< 74 or > 83) was found to be associated with a significant change in the BPD measurement expected for any given age. Using the CI, certain variations in the shape of the fetal skull, such as dolichocephaly (CI < 74) and brachycephaly (CI > 84), have been described.12
Gray and collaborators10 proposed that the CI varies with advancing age (Table 3–5). These authors also suggested that the normal CI range is within 1 SD of the mean, with a sensitivity of 84% and a false-positive rate of 35% for detection of a misleading BPD value.
| Week | Mean Cephalic Index | –1 SD | +1 SD |
|---|---|---|---|
| 14 | 81.5 | 77.8 | 85.3 |
| 15 | 81.0 | 77.3 | 84.8 |
| 16 | 80.5 | 76.8 | 84.3 |
| 17 | 80.1 | 76.4 | 83.9 |
| 18 | 79.7 | 76.0 | 83.5 |
| 19 | 79.4 | 75.7 | 83.2 |
| 20 | 79.1 | 75.4 | 82.9 |
| 21 | 78.8 | 75.1 | 82.6 |
| 22 | 78.6 | 74.9 | 82.4 |
| 23 | 78.4 | 74.7 | 82.2 |
| 24 | 78.3 | 74.6 | 82.0 |
| 25 | 78.1 | 74.4 | 81.9 |
| 26 | 78.0 | 74.3 | 81.8 |
| 27 | 78.0 | 74.3 | 81.8 |
| 28 | 78.0 | 74.3 | 81.8 |
| 29 | 78.0 | 74.3 | 81.8 |
| 30 | 78.1 | 74.4 | 81.9 |
| 31 | 78.2 | 74.5 | 82.0 |
| 32 | 78.3 | 74.6 | 82.1 |
| 33 | 78.5 | 74.8 | 82.3 |
| 34 | 78.7 | 75.0 | 82.5 |
| 35 | 78.9 | 75.2 | 82.7 |
| 36 | 79.2 | 75.5 | 83.0 |
| 37 | 79.5 | 75.8 | 83.3 |
| 38 | 79.9 | 76.2 | 83.7 |
| 39 | 80.3 | 76.6 | 84.1 |
| 40 | 80.7 | 77.0 | 84.5 |
The head/abdominal circumference ratio was used to determine growth restriction in fetuses.15
The HC represents the outer perimeter of the fetal calvarium measured at the level of the BPD.
The correct anatomical plane that should be used to measure the HC corresponds to the axial plane described by Campbell and Thoms,6 previously delineated in the section on BPD. Ideally, the formula for the circumference of an ellipse should be used.16
Both transverse and longitudinal diameters should be outer-to-outer distances (as for the CI). Adequate calculation of the HC can also be made using the formula17
The HC can also be measured along the outer perimeter of the calvarium, using a map measurer or electronic digitizer, as originally described by Hadlock’s group.17,18 The calculation of a circumference from two diameters is equivalent to the electronically digitized perimeter measurement, although the probability of technical errors between these techniques exists.
The HC was originally described as a useful index to estimate fetal age in cases in which variations in head shape (eg, dolichocephaly or brachycephaly) adversely affected the accuracy of the BPD.19 Several authors have demonstrated that the HC is a better predictor of fetal age than the BPD.20,21,22 This is because the HC is more shape independent, and thus is less prone to be affected by molding of the fetal head (Tables 3–6 and 3–7).
| Menstrual Age (weeks) | Head Circumference (cm) | Menstrual Age (weeks) | Head Circumference (cm) |
|---|---|---|---|
| 12.0 | 6.8 | 26.5 | 25.1 |
| 12.5 | 7.5 | 27.0 | 25.6 |
| 13.0 | 8.2 | 27.5 | 26.1 |
| 13.5 | 8.9 | 28.0 | 26.6 |
| 14.0 | 9.7 | 28.5 | 27.1 |
| 14.5 | 10.4 | 29.0 | 27.5 |
| 15.0 | 11.0 | 29.5 | 28.0 |
| 15.5 | 11.7 | 30.0 | 28.4 |
| 16.0 | 12.4 | 30.5 | 28.8 |
| 16.5 | 13.1 | 31.0 | 29.3 |
| 17.0 | 13.8 | 31.5 | 29.7 |
| 17.5 | 14.4 | 32.0 | 30.1 |
| 18.0 | 15.1 | 32.5 | 30.4 |
| 18.5 | 15.8 | 33.0 | 30.8 |
| 19.0 | 16.4 | 33.5 | 31.2 |
| 19.5 | 17.0 | 34.0 | 31.5 |
| 20.0 | 17.7 | 34.5 | 31.8 |
| 20.5 | 18.3 | 35.0 | 32.2 |
| 21.0 | 18.9 | 35.5 | 32.5 |
| 21.5 | 19.5 | 36.0 | 32.8 |
| 22.0 | 20.1 | 36.5 | 33.0 |
| 22.5 | 20.7 | 37.0 | 33.3 |
| 23.0 | 21.3 | 37.5 | 33.5 |
| 23.5 | 21.9 | 38.0 | 33.8 |
| 24.0 | 22.4 | 38.5 | 34.0 |
| 24.5 | 23.0 | 39.0 | 34.2 |
| 25.0 | 23.5 | 39.5 | 34.4 |
| 25.5 | 24.1 | 40.0 | 34.6 |
| 26.0 | 24.6 |
| Gestational Age (weeks + days) | |||
|---|---|---|---|
| Head Perimeter (mm) | 5th Percentile | 50th Percentile | 95th Percentile |
| 80 | 10 + 5 | 12 + 4 | 14 + 2 |
| 84 | 11 + 0 | 12 + 5 | 14 + 4 |
| 88 | 11 + 2 | 13 + 0 | 14 + 6 |
| 92 | 11 + 4 | 13 + 2 | 15 + 0 |
| 96 | 11 + 6 | 13 + 4 | 15 + 2 |
| 100 | 12 + 1 | 13 + 6 | 15 + 4 |
| 104 | 12 + 3 | 14 + 1 | 15 + 6 |
| 108 | 12 + 5 | 14 + 3 | 16 + 1 |
| 112 | 13 + 0 | 14 + 5 | 16 + 3 |
| 116 | 13 + 2 | 15 + 0 | 16 + 5 |
| 120 | 13 + 4 | 15 + 2 | 17 + 0 |
| 124 | 13 + 6 | 15 + 4 | 17 + 2 |
| 128 | 14 + 1 | 15 + 6 | 17 + 5 |
| 132 | 14 + 3 | 16 + 1 | 18 + 0 |
| 136 | 14 + 5 | 16 + 4 | 18 + 2 |
| 140 | 15 + 0 | 16 + 6 | 18 + 4 |
| 144 | 15 + 3 | 17 + 1 | 18 + 6 |
| 148 | 15 + 5 | 17 + 3 | 19 + 2 |
| 152 | 16 + 0 | 17 + 6 | 19 + 4 |
| 156 | 16 + 3 | 18 + 1 | 19 + 6 |
| 160 | 16 + 5 | 18 + 3 | 20 + 1 |
| 164 | 17 + 0 | 18 + 6 | 20 + 4 |
| 168 | 17 + 3 | 19 + 1 | 20 + 6 |
| 172 | 17 + 5 | 19 + 3 | 21 + 2 |
| 176 | 18 + 0 | 19 + 6 | 21 + 4 |
| 180 | 18 + 3 | 20 + 1 | 21 + 6 |
| 184 | 18 + 5 | 20 + 4 | 22 + 2 |
| 188 | 19 + 1 | 20 + 6 | 22 + 4 |
| 192 | 19 + 3 | 21 + 2 | 23 + 0 |
| 196 | 19 + 6 | 21 + 4 | 23 + 3 |
| 200 | 20 + 2 | 22 + 0 | 23 + 5 |
| 204 | 20 + 4 | 22 + 2 | 24 + 1 |
| 208 | 21 + 0 | 22 + 5 | 24 + 3 |
| 212 | 21 + 2 | 23 + 1 | 24 + 6 |
| 216 | 21 + 5 | 23 + 3 | 25 + 2 |
| 220 | 22 + 1 | 23 + 6 | 25 + 4 |
| 224 | 22 + 4 | 24 + 2 | 26 + 0 |
| 228 | 22 + 6 | 24 + 5 | 26 + 3 |
| 232 | 23 + 2 | 25 + 0 | 26 + 6 |
| 236 | 23 + 5 | 25 + 3 | 27 + 2 |
| 240 | 24 + 1 | 25 + 6 | 27 + 4 |
| 244 | 24 + 4 | 26 + 2 | 28 + 0 |
| 248 | 25 + 0 | 26 + 5 | 28 + 3 |
| 252 | 25 + 3 | 27 + 1 | 28 + 6 |
| 256 | 25 + 6 | 27 + 4 | 29 + 2 |
| 260 | 26 + 1 | 28 + 0 | 29 + 5 |
| 264 | 26 + 4 | 28 + 3 | 30 + 1 |
| 268 | 27 + 1 | 28 + 6 | 30 + 4 |
| 272 | 27 + 4 | 29 + 2 | 31 + 0 |
| 276 | 28 + 0 | 29 + 5 | 31 + 3 |
| 280 | 28 + 3 | 30 + 1 | 31 + 6 |
| 284 | 28 + 6 | 30 + 4 | 32 + 2 |
| 288 | 29 + 2 | 31 + 0 | 32 + 6 |
| 292 | 29 + 5 | 31 + 4 | 33 + 2 |
| 296 | 30 + 1 | 32 + 0 | 33 + 5 |
| 300 | 30 + 5 | 32 + 3 | 34 + 1 |
| 304 | 31 + 1 | 32 + 6 | 34 + 5 |
| 308 | 31 + 4 | 33 + 3 | 35 + 1 |
| 312 | 32 + 1 | 33 + 6 | 35 + 4 |
| 316 | 32 + 4 | 34 + 2 | 36 + 0 |
| 320 | 33 + 0 | 34 + 6 | 36 + 4 |
| 324 | 33 + 4 | 35 + 2 | 37 + 0 |
| 328 | 34 + 0 | 35 + 5 | 37 + 4 |
| 332 | 34 + 4 | 36 + 2 | 38 + 0 |
| 336 | 35 + 0 | 36 + 5 | 38 + 4 |
| 340 | 35 + 3 | 37 + 2 | 39 + 0 |
| 344 | 36 + 0 | 37 + 5 | 39 + 4 |
| 348 | 36 + 4 | 38 + 2 | 40 + 0 |
| 352 | 37 + 0 | 38 + 5 | 40 + 4 |
| 356 | 37 + 4 | 39 + 2 | 41 + 0 |
| 360 | 38 + 0 | 39 + 6 | 41 + 4 |
| 364 | 38 + 4 | 40 + 2 | 42 + 1 |
As with other parameters, such as the BPD, the variability for estimation of age via the HC progressively increases with advancing menstrual age, ranging from ±1.19 at the 12- to 18-week interval to ±2.70 at the 36- to 42-week interval (Table 3–8).9
| Fetal Parameters | Subgroup Variability (± 2 SD) in Weeks | ||||
|---|---|---|---|---|---|
| 12–18 Weeks | 18–24 Weeks | 24–30 Weeks | 30–36 Weeks | 36–42 Weeks | |
| Biparietal diameter | ±1.19 | ±1.73 | ±2.18 | ±3.08 | ±3.20 |
| Head circumference | ±1.19 | ±1.48 | ±2.06 | ±2.98 | ±2.70 |
The HC has also been used in equations developed to estimate fetal weight,23 to monitor normal fetal growth as well as intrauterine growth restriction,24,25 and for the diagnosis of microcephaly (Table 3–9). Microcephaly is a clinical syndrome characterized by an abnormally small head. Most authors agree that the diagnosis of microcephaly should be suspected when the HC is 3 SD below the mean for a given gestational age.26,27,28 Nevertheless, the antenatal accuracy for the diagnosis of microcephaly using this parameter has been reported to be as low as 56.2%.28 To overcome this low performance, other measurements, such as the frontal lobe distance and the thalamic frontal lobe distance, have been proposed.29 Other typical features of microcephalic fetuses, such as a cone-shaped head, a large face, large ears, and a sloping forehead (“birdhead”),29 as well as associated anomalies such as ventricular enlargement, porencephaly, and cephaloceles,28 should be carefully searched. Some investigators have postulated that a reduction in blood supply to the underdeveloped cerebral hemispheres in fetuses with microcephaly could be detected with color Doppler and power Doppler imaging.30
| Week | Mean | Head Circumference (mm): SD Below Mean | ||||
|---|---|---|---|---|---|---|
| –1 | –2 | –3 | –4 | –5 | ||
| 20 | 175 | 160 | 145 | 131 | 116 | 101 |
| 21 | 187 | 172 | 157 | 143 | 128 | 113 |
| 22 | 198 | 184 | 169 | 154 | 140 | 125 |
| 23 | 210 | 195 | 180 | 166 | 151 | 136 |
| 24 | 221 | 206 | 191 | 177 | 162 | 147 |
| 25 | 232 | 217 | 202 | 188 | 173 | 158 |
| 26 | 242 | 227 | 213 | 198 | 183 | 169 |
| 27 | 252 | 238 | 223 | 208 | 194 | 179 |
| 28 | 262 | 247 | 233 | 218 | 203 | 189 |
| 29 | 271 | 257 | 242 | 227 | 213 | 198 |
| 30 | 281 | 266 | 251 | 236 | 222 | 207 |
| 31 | 289 | 274 | 260 | 245 | 230 | 216 |
| 32 | 297 | 283 | 268 | 253 | 239 | 224 |
| 33 | 305 | 290 | 276 | 261 | 246 | 232 |
| 34 | 312 | 297 | 283 | 268 | 253 | 239 |
| 35 | 319 | 304 | 289 | 275 | 260 | 245 |
| 36 | 325 | 310 | 295 | 281 | 266 | 251 |
| 37 | 330 | 316 | 301 | 286 | 272 | 257 |
| 38 | 335 | 320 | 306 | 291 | 276 | 262 |
| 39 | 339 | 325 | 310 | 295 | 281 | 266 |
| 40 | 343 | 328 | 314 | 299 | 284 | 270 |
| 41 | 346 | 331 | 316 | 302 | 287 | 272 |
| 42 | 348 | 333 | 319 | 304 | 289 | 275 |
As the nomograms for HC require knowledge of the accurate fetal age, and this information is not always available, the use of ratios such as the HC-to-femur length ratios has the advantage of reducing dependence on reliable age evaluation (Table 3–10).28 This ratio assumes that limb growth is not affected in fetuses with microcephaly, which might not always be the case.
| Week | Mean | SD Below Mean | ||||
|---|---|---|---|---|---|---|
| –5 | –4 | –3 | –2 | –1 | ||
| 20 | 0.180 | 0.107 | 0.122 | 0.137 | 0.152 | 0.167 |
| 21 | 0.190 | 0.111 | 0.126 | 0.141 | 0.156 | 0.171 |
| 22 | 0.190 | 0.115 | 0.130 | 0.145 | 0.160 | 0.175 |
| 23 | 0.190 | 0.118 | 0.133 | 0.148 | 0.163 | 0.178 |
| 24 | 0.200 | 0.121 | 0.136 | 0.151 | 0.166 | 0.181 |
| 25 | 0.200 | 0.123 | 0.138 | 0.153 | 0.168 | 0.183 |
| 26 | 0.200 | 0.125 | 0.140 | 0.155 | 0.170 | 0.185 |
| 27 | 0.200 | 0.127 | 0.142 | 0.157 | 0.172 | 0.187 |
| 28 | 0.200 | 0.129 | 0.144 | 0.159 | 0.174 | 0.189 |
| 29 | 0.200 | 0.130 | 0.145 | 0.160 | 0.175 | 0.190 |
| 30 | 0.210 | 0.131 | 0.146 | 0.161 | 0.176 | 0.191 |
| 31 | 0.210 | 0.132 | 0.147 | 0.162 | 0.177 | 0.192 |
| 32 | 0.210 | 0.134 | 0.149 | 0.164 | 0.179 | 0.194 |
| 33 | 0.210 | 0.135 | 0.150 | 0.165 | 0.180 | 0.195 |
| 34 | 0.210 | 0.136 | 0.151 | 0.166 | 0.181 | 0.196 |
| 35 | 0.210 | 0.138 | 0.153 | 0.168 | 0.183 | 0.198 |
| 36 | 0.210 | 0.140 | 0.155 | 0.170 | 0.185 | 0.200 |
| 37 | 0.220 | 0.142 | 0.157 | 0.172 | 0.187 | 0.202 |
| 38 | 0.220 | 0.144 | 0.159 | 0.174 | 0.189 | 0.204 |
| 39 | 0.220 | 0.147 | 0.162 | 0.177 | 0.192 | 0.207 |
| 40 | 0.230 | 0.151 | 0.166 | 0.181 | 0.196 | 0.211 |
| 41 | 0.230 | 0.155 | 0.170 | 0.185 | 0.200 | 0.215 |
| 42 | 0.230 | 0.160 | 0.175 | 0.190 | 0.205 | 0.220 |
ORBITAL DIAMETERS: INNER ORBITAL DIAMETER, OUTER ORBITAL DIAMETER, AND OCULAR DIAMETER
The inner orbital, or interorbital, diameter (IOD) is defined as the distance between the medial border of one orbit and the medial border of the opposite orbit. The outer orbital, or biorbital, diameter (OOD) is defined as the distance between the lateral border of one orbit and the lateral border of the opposite orbit.31 The ocular diameter corresponds to the distance between the medial border and the lateral border of a single orbit.
To measure the orbital diameters, an axial plane slightly caudad to the BPD is commonly used (Figure 3–3). The criteria used for this section are (1) a symmetrical section, (2) both eyes imaged and of equal diameter, and (3) the largest possible diameter of the eyes.32 Measurements of orbital diameters can also be accomplished using a coronal plane, ~2 cm posterior to the glabella-alveolar line.31
The study of the orbital diameters should help in the diagnosis of hypotelorism, hypertelorism, and microphthalmos. Trout and colleagues33 have found that in cases of hypotelorism, both the IOD and the OOD clearly fall below 2 SD of the mean. In cases of hypertelorism, the IOD fell above the 95th percentile, whereas the OOD measurement fell within normal limits but near the 95th percentile. Hypotelorism has been associated with chromosomal anomalies (trisomy 13, trisomy 21, 18p–, 5p–, and 14q +), holoprosencephaly, Meckel-Gruber syndrome, microcephaly, maternal phenylketonuria, and others.32,33,34 Hypertelorism can occur as an isolated defect but has also been associated with a long list of malformations and syndromes, such as frontal, ethmoidal, or sphenoidal meningoencephalocele; median cleft syndrome; and craniosynostosis.34
Mayden and associates31 developed nomograms of orbital measurements as a function of the BPD, which are taken as a classical reference to which newer data are compared (Table 3–11). Nomograms for orbital diameters as a function of age have been published because the authors33 felt that some patients could have pathologic intracranial conditions that might alter the BPD, making this measurement less reliable (Table 3–12).
| Biparietal Diameter (cm) | Inner Orbital Diameter (cm) | Outer Orbital Diameter (cm) |
|---|---|---|
| 1.9 | 0.5 | 1.3 |
| 2.0 | 0.5 | 1.4 |
| 2.1 | 0.6 | 1.5 |
| 2.2 | 0.6 | 1.6 |
| 2.3 | 0.6 | 1.7 |
| 2.4 | 0.7 | 1.7 |
| 2.5 | 0.7 | 1.8 |
| 2.6 | 0.7 | 1.9 |
| 2.7 | 0.8 | 2.0 |
| 2.8 | 0.8 | 2.1 |
| 2.9 | 0.8 | 2.1 |
| 3.0 | 0.9 | 2.2 |
| 3.1 | 0.9 | 2.3 |
| 3.2 | 0.9 | 2.4 |
| 3.3 | 1.0 | 2.5 |
| 3.4 | 1.0 | 2.5 |
| 3.5 | 1.0 | 2.6 |
| 3.6 | 1.0 | 2.7 |
| 3.7 | 1.1 | 2.7 |
| 3.8 | 1.1 | 2.8 |
| 4.0 | 1.2 | 3.0 |
| 4.2 | 1.2 | 3.1 |
| 4.3 | 1.2 | 3.2 |
| 4.4 | 1.3 | 3.2 |
| 4.5 | 1.3 | 3.3 |
| 4.6 | 1.3 | 3.4 |
| 4.7 | 1.3 | 3.4 |
| 4.8 | 1.4 | 3.5 |
| 4.9 | 1.4 | 3.6 |
| 5.0 | 1.4 | 3.6 |
| 5.1 | 1.4 | 3.7 |
| 5.2 | 1.4 | 3.8 |
| 5.3 | 1.5 | 3.8 |
| 5.4 | 1.5 | 3.9 |
| 5.5 | 1.5 | 4.0 |
| 5.6 | 1.5 | 4.0 |
| 5.7 | 1.5 | 4.1 |
| 5.8 | 1.6 | 4.1 |
| 5.9 | 1.6 | 4.2 |
| 6.0 | 1.6 | 4.3 |
| 6.1 | 1.6 | 4.3 |
| 6.2 | 1.6 | 4.4 |
| 6.3 | 1.7 | 4.4 |
| 6.4 | 1.7 | 4.5 |
| 6.5 | 1.7 | 4.5 |
| 6.6 | 1.7 | 4.6 |
| 6.7 | 1.7 | 4.6 |
| 6.8 | 1.7 | 4.7 |
| 6.9 | 1.7 | 4.7 |
| 7.0 | 1.8 | 4.8 |
| 7.1 | 1.8 | 4.8 |
| 7.3 | 1.8 | 4.9 |
| 7.4 | 1.8 | 5.0 |
| 7.5 | 1.8 | 5.0 |
| 7.6 | 1.8 | 5.1 |
| 7.7 | 1.8 | 5.1 |
| 7.8 | 1.8 | 5.2 |
| 7.9 | 1.9 | 5.2 |
| 8.0 | 1.9 | 5.3 |
| 8.2 | 1.9 | 5.4 |
| 8.3 | 1.9 | 5.4 |
| 8.4 | 1.9 | 5.4 |
| 8.5 | 1.9 | 5.5 |
| 8.6 | 1.9 | 5.5 |
| 8.8 | 1.9 | 5.6 |
| 8.9 | 1.9 | 5.6 |
| 9.0 | 1.9 | 5.7 |
| 9.1 | 1.9 | 5.7 |
| 9.2 | 1.9 | 5.8 |
| 9.3 | 1.9 | 5.8 |
| 9.4 | 1.9 | 5.8 |
| 9.6 | 1.9 | 5.9 |
| 9.7 | 1.9 | 5.9 |
| Gestational Age (weeks) | Inner Orbital Diameter (mm) | Outer Orbital Diameter (mm) | ||||
|---|---|---|---|---|---|---|
| 5th Percentile | 50th Percentile | 95th Percentile | 5th Percentile | 50th Percentile | 95th Percentile | |
| 13 | 4 | 7 | 10 | 12 | 16 | 20 |
| 14 | 5 | 8 | 11 | 14 | 18 | 22 |
| 15 | 5 | 8 | 11 | 17 | 21 | 25 |
| 16 | 6 | 9 | 12 | 19 | 23 | 27 |
| 17 | 7 | 10 | 13 | 21 | 25 | 29 |
| 18 | 8 | 11 | 14 | 24 | 27 | 31 |
| 19 | 8 | 11 | 14 | 26 | 30 | 34 |
| 20 | 9 | 12 | 15 | 28 | 32 | 36 |
| 21 | 10 | 13 | 16 | 30 | 34 | 38 |
| 22 | 10 | 13 | 16 | 32 | 36 | 40 |
| 23 | 11 | 14 | 17 | 33 | 37 | 41 |
| 24 | 12 | 14 | 17 | 35 | 39 | 43 |
| 25 | 12 | 15 | 18 | 37 | 41 | 45 |
| 26 | 13 | 16 | 19 | 39 | 43 | 47 |
| 27 | 13 | 16 | 19 | 40 | 44 | 48 |
| 28 | 14 | 17 | 20 | 42 | 46 | 50 |
| 29 | 14 | 17 | 20 | 43 | 47 | 51 |
| 30 | 15 | 18 | 21 | 45 | 49 | 52 |
| 31 | 15 | 18 | 21 | 46 | 50 | 54 |
| 32 | 16 | 19 | 22 | 47 | 51 | 55 |
| 33 | 17 | 20 | 23 | 48 | 52 | 56 |
| 34 | 17 | 20 | 23 | 49 | 53 | 57 |
| 35 | 18 | 21 | 24 | 50 | 54 | 58 |
Microphthalmos can be suspected when the orbital diameter falls below the fifth percentile for age (Table 3–13). Because this is a statistical definition, careful examination of the intraorbital anatomy, as well as detection of associated anomalies, is warranted. Conditions associated with microphthalmos include chromosomal (trisomies 13 and 18 and trisomy 9 mosaic), environmental (fetal toxoplasmosis, rubella, varicella, and alcohol syndrome and maternal phenylketonuria), and multiple syndromes (eg, frontonasal dysplasia, Fraser syndrome, Lenz syndrome, and Fanconi syndrome).35
| Gestational Age (weeks) | Ocular Diameter (mm) | ||
|---|---|---|---|
| 5th Percentile | 50th Percentile | 95th Percentile | |
| 11 | — | — | — |
| 12 | 1 | 3 | 6 |
| 13 | 2 | 4 | 7 |
| 14 | 3 | 5 | 8 |
| 15 | 4 | 6 | 9 |
| 16 | 5 | 7 | 9 |
| 17 | 5 | 8 | 10 |
| 18 | 6 | 9 | 11 |
| 19 | 7 | 9 | 12 |
| 20 | 8 | 10 | 13 |
| 21 | 8 | 11 | 13 |
| 22 | 9 | 12 | 14 |
| 23 | 10 | 12 | 15 |
| 24 | 10 | 13 | 15 |
| 25 | 11 | 13 | 16 |
| 26 | 12 | 14 | 16 |
| 27 | 12 | 14 | 17 |
| 28 | 13 | 15 | 17 |
| 29 | 13 | 15 | 18 |
| 30 | 14 | 16 | 18 |
| 31 | 14 | 16 | 19 |
| 32 | 14 | 17 | 19 |
| 33 | 15 | 17 | 19 |
| 34 | 15 | 17 | 20 |
| 35 | 15 | 18 | 20 |
| 36 | 16 | 18 | 20 |
| 37 | 16 | 18 | 21 |
| 38 | 16 | 18 | 21 |
| 39 | 16 | 19 | 21 |
| 40 | 16 | 19 | 21 |
The lateral ventricular width–hemispheric width (LVW/HW) ratio represents the percentage of the whole cerebral hemisphere that corresponds to the lateral ventricle, measured in an axial plane.
The widths of the lateral ventricles and the hemispheres are measured in a plane parallel to the BPD, but slightly closer to the top of the head (Figure 3–4). In this section, the lateral ventricles (LVs) appear as two linear echoes, roughly parallel to the midline. The LVW is then measured as the distance from the midline to the first echoes of the LVs. This measurement is thus slightly greater than that of the actual LV, because it does not use its medial wall as the internal landmark. The HW is the largest distance between the midline and the inner edge of the skull, measured perpendicular to the midline. To avoid tilting errors, one should be able to recognize both LVs as being equal in size.36
The normal LVW/HW ratio at 15 weeks can be as high as 71%, with a mean of 56% and a range of 40% to 71%. By 37 postmenstrual weeks, the mean is 29%, with a range of 24% to 34% (Table 3–14). These data reflect the rapid growth of the cerebral hemispheres as pregnancy progresses, making the LVW/HW decrease with advancing age.37
| Menstrual Age (weeks) | Lateral Ventricular Width (LVW) (cm) | Hemispheric Width (HW) (cm) | Ratio LVW/HW (% ± 2 SD) |
|---|---|---|---|
| 15 | 0.75 | 1.4 | 56 (40–71) |
| 16 | 0.86 | 1.5 | 57 (45–69) |
| 17 | 0.85 | 1.5 | 52 (42–62) |
| 18 | 0.83 | 1.8 | 46 (40–52) |
| 19 | — | — | — |
| 20 | 0.82 | 1.9 | 43 (29–57) |
| 21 | 0.76 | 2.2 | 35 (27–43) |
| 22 | 0.82 | 2.6 | 32 (26–38) |
| 23 | 0.83 | 2.5 | 33 (24–42) |
| 24 | 0.83 | 2.7 | 31 (23–39) |
| 25 | 1.1 | 3.0 | 34 (26–42) |
| 26 | 0.9 | 3.0 | 30 (24–36) |
| 27 | 0.9 | 3.0 | 28 (23–34) |
| 28 | 1.1 | 3.3 | 31 (18–45) |
| 29 | 1.0 | 3.4 | 29 (22–37) |
| 30 | 1.0 | 3.4 | 30 (26–34) |
| 31 | 1.0 | 3.4 | 29 (23–36) |
| 32 | 1.1 | 3.6 | 31 (26–36) |
| 33 | 1.1 | 3.4 | 31 (25–37) |
| 34 | 1.1 | 3.8 | 28 (23–33) |
| 35 | 1.1 | 3.8 | 29 (26–31) |
| 36 | 1.1 | 3.9 | 28 (23–34) |
| 37 | 1.2 | 4.1 | 29 (24–34) |
| Term | 1.2 | 4.3 | 28 (22–33) |
The LVW/HW ratio was developed to monitor ventricular growth in an attempt to provide an early diagnosis of hydrocephaly at a time when the classical diagnosis of intrauterine hydrocephaly relied on the finding of a BPD > 11 cm or a head-to-abdomen ratio > 2.38 Although this ratio provided a way to diagnose hydrocephaly up to 2 months before the BPD was pathologically enlarged,36 other parameters, such as the width of the lateral ventricular atrium were able to do that too.37 Transvaginal neurosonography can evaluate the fetal ventricular system in a much more accurate way and somewhat earlier.
One of the major drawbacks of the LVW/HW ratio is that echogenic “lines” previously used to delineate the ventricular walls are instead reflections from small venous structures deep in the fetal white matter.39 Also, the wide SD of this ratio renders it ineffective for detecting early ventricular dilation.40
The anterior horn corresponds to the portion of the lateral ventricles anterior to the interventricular foramen.
The cavum septi pellucidi (CSP) is a closed cavity in the brain, located on the midline of the transverse plane between the two leaves of septum pellucidum, which separate the lateral ventricles.
To allow proper visualization of the anterior horn with transabdominal sonography, a horizontal (axial) scanning plane parallel and slightly anterior to that for the BPD should be used (Figure 3–5). In this section one should be able to recognize, in an anterior-to-posterior fashion, the anterior horns (AHs), the CSP, and the atria of the lateral ventricles (A). The cerebrofrontal horn distance (CFHD) can then be measured from the midline echo to the lateral wall of the anterior horn distal to the transducer. It should be kept in mind that from about 30 postmenstrual weeks on, the lumen of the anterior horns is very hard to visualize, and only its lateral aspect is defined. The frontal HW is measured from the leading edge of the midline echo to the inner aspect of the fetal calvarium at the point of maximal HW.41
The relative size of the anterior horns decreases with advancing gestational age. This was demonstrated by Goldstein and coworkers,41 who found that, in spite of an increasing CFHD throughout pregnancy, the CFHD/HW ratio decreases throughout gestation, from 50% at 15 menstrual weeks to 28% at term (Tables 3–15 and 3–16). These findings correlate with previous reports42 and are related to the fact that the internal lumina of the ventricles are progressively reduced and molded by the growth of the basal nuclei, the corpus striatum, and the knee of the corpus callosum.
| Gestational Age (weeks) | Mean ± 2 SD | Percentile | ||
|---|---|---|---|---|
| 10th | 50th | 90th | ||
| 15 | 0.7 ± 0.10 | 0.6 | 0.7 | 0.9 |
| 16 | 0.8 ± 0.12 | 0.5 | 0.8 | 1.0 |
| 17 | 0.9 ± 0.16 | 0.7 | 0.9 | 1.2 |
| 18 | 0.8 ± 0.05 | 0.8 | 0.9 | 0.9 |
| 19 | 0.8 ± 0.10 | 0.7 | 0.8 | 0.9 |
| 20 | 0.8 ± 0.10 | 0.7 | 0.8 | 1.0 |
| 21 | 0.8 ± 0.08 | 0.7 | 0.8 | 0.9 |
| 22 | 0.8 ± 0.07 | 0.7 | 0.8 | 0.9 |
| 23 | 0.9 ± 0.10 | 0.7 | 0.8 | 1.0 |
| 24 | 0.8 ± 0.07 | 0.7 | 0.9 | 0.9 |
| 25 | 0.8 ± 0.05 | 0.7 | 0.8 | 0.8 |
| 26 | 0.9 ± 0.11 | 0.8 | 1.0 | 1.1 |
| 27 | 1.0 ± 0.17 | 0.8 | 1.0 | 1.2 |
| 28 | 0.9 ± 0.13 | 0.8 | 0.9 | 1.1 |
| 29 | 1.0 ± 0.09 | 0.9 | 1.0 | 1.1 |
| 30 | 1.0 ± 0.12 | 0.8 | 1.0 | 1.2 |
| 31 | 1.0 ± 0.16 | 0.8 | 1.0 | 1.3 |
| 32 | 1.1 ± 0.08 | 1.0 | 1.1 | 1.2 |
| 33 | 1.1 ± 0.12 | 0.9 | 1.1 | 1.2 |
| 34 | 1.1 ± 0.13 | 0.9 | 1.0 | 1.2 |
| 35 | 1.2 ± 0.15 | 1.0 | 1.1 | 1.4 |
| 36 | 1.2 ± 0.10 | 1.1 | 1.2 | 1.3 |
| 37 | 1.1 ± 0.00 | 1.1 | 1.1 | 1.1 |
| 38 | 1.2 ± 0.06 | 1.2 | 1.2 | 1.3 |
| 39 | 1.3 ± 0.19 | 1.0 | 1.3 | 1.4 |
| 40 | 1.2 ± 0.00 | 1.2 | 1.2 | 1.2 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree