Sonographic evaluation of the intestine is increasing in popularity due to its safety, noninvasive nature, accessibility, and high acceptability by patients. It is now recognized as one of the most valuable imaging modalities in the assessment of patients with inflammatory bowel disease. In addition, recent technical advances in ultrasound (US), especially contrast-enhanced US and shear wave elastography, have given US a competitive edge allowing for subjective and objective measurements of mural and mesenteric inflammation. The dynamic performance and high resolution of US allow for functional and morphologic assessment of the bowel, making it a desirable technique.
Key points
- •
High-resolution ultrasound (US) allows for excellent detection of bowel pathology and its complications.
- •
Multiple publications have demonstrated that US is comparable in performance to computed tomography and MR imaging for the assessment of inflammatory bowel disease, supporting the choice of US as a first-line investigation.
- •
US is sensitive in the detection of mural and mesenteric inflammation allowing for a subjective and objective assessment of disease activity and therapeutic response that can be followed overtime.
- •
New US techniques such as contrast-enhanced ultrasound and elastography are now providing an objective evaluation of the mural perfusion and intestinal stiffness, important in the evaluation of strictures.
Video content accompanies this article at http://www.radiologic.theclinics.com .
Introduction
Sonographic evaluation of the intestine is rapidly growing in popularity due to its safety, noninvasive nature, accessibility, and high acceptance by patients. Bowel or intestinal ultrasound is increasingly recognized as a valuable imaging modality for the evaluation of patients with inflammatory bowel disease (IBD) where frequent examinations are needed to monitor disease and therapeutic response. Today, disease management has changed from treating patient’s symptoms to recognition that surveillance imaging regarding symptomatology should be the preferred standard of care. This acknowledges that patients’ symptoms are often not a reflection of their disease. , Specially, in IBD, the young age of onset, the chronic nature of the disease, its significant complications, and fluctuating nature of the disease make this radiation-free technique a particularly desirable and important imaging modality. In addition, ultrasound (US) can evaluate all small bowel segments including those proximal loops not assessed by endoscopy and shows the terminal ileum (TI) in those with failed intubation at endoscopy.
Current state-of-the-art US equipment allows for exceptional detailed evaluation of the bowel wall, mesentery, and surrounding peritoneum. The spatial resolution in grayscale US permits detailed identification of the normal bowel wall layers, without requirement for contrast enhancement to detect subtle transmural and mesenteric changes.
Real-time performance gives an advantage to the US operator to directly communicate with the patient regarding ongoing and acute symptomatology often influencing the examination performance. For instance, correlation of an identifiable sonographic abnormality with a focal area of tenderness directs the remaining of the examination. One of the most desirable advantages of US over other imaging techniques is its dynamic capability. This allows for the assessment of bowel motility, compliance, detection of fixed or transient changes, and recognition of dysfunctional peristalsis, all important observations as diagnotic clues for disease. For instance, the detection of fluid-filled dysfunctional loops of peristaltic bowel in a patient with bloating will direct the search for the cause of obstruction.
Detection of mural inflammation is vital for the assessment of disease activity in IBD. Color Doppler imaging (CDI) displays mural and mesenteric vascularity. However, CDI only detects large vessels with fast flow and may be compromised by obesity and deep position of abnormal segments causing poor flow detection. Therefore, it is advocated that contrast-enhanced ultrasound (CEUS) is a necessary addition to brightness mode (B-mode) imaging in these scenarios. CEUS allows for mural blood flow detection at the microcirculatory level and provides objective measurements of mural flow that can be followed overtime for disease activity assessment and therapeutic response. ,
In addition, the increasing popularity of shear wave elastography (SWE) for the evaluation of tissue stiffness has sparked interest to use this technique in the assessment of strictures in IBD. , Strictures are characterized by the presence of inflammation, muscular hypertrophy, and fibrotic components in various degrees. In combination, these US techniques provide all the necessary tools for stricture characterization.
Although sonographic evaluation predominantly focused on changes of the small and large bowel, it may be also expand to evaluate for perianal disease for detection of fistulas and inflammatory masses with great resolution.
The importance of bowel US goes beyond IBD. The US has long been used as the first-line imaging modality for the evaluation of abdominal pain in the acute setting to detect common conditions such as appendicitis, diverticulitis, or enteritis. However, most routine abdominal-pelvic US examinations have focused on the solid organs and in most cases excluded the bowel due to the traditional view that gas does not allow for intestinal visualization. The authors prefer that this practice should be improved with the addition of a quadrant survey to detect any potential abnormal bowel segment that may direct the need for a more detailed bowel assessment.
Normal anatomy and imaging techniques
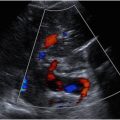
Conventional grayscale US, with high-resolution imaging, shows normal bowel wall as a multilayered cylindrical structure, which shows a diagnostic gut signature with 5 layers that alternate in echogenicity with the recognition that the hypoechoic (black) layers represent muscular components. From the lumen to the outer layer, they are as follows: (1) mucosa—echogenic, (2) muscularis mucosa—hypoechoic (not always visible or very thin), (3) submucosa—echogenic (most obviously recognized), (4) muscularis propria—hypoechoic (well seen), (5) serosa or adventitia—hyperechoic (normally difficult to visualize; Fig. 1 A–D ).

The exceptional depiction of the wall layers by US gives this modality an advantage over other cross-sectional imaging techniques that require contrast enhancement for bowel layer differentiation. Visualization of the gut signature may vary depending on the transducer resolution and body habitus. Normal bowel is compliant and easy to compress during the sonographic evaluation, with wall measuring less than 3 mm. The normal colonic wall may vary in thickness depending on the degree of colonic distension with normal average thickness of less than 3 mm if well-distended and less than 5 mm if not.
Specific features to aid in identifying a bowel segment include recognition of rugae in the stomach, valvulae conniventes in the small bowel, and haustra in the colon.
Normal mural vascularity of the bowel is evaluated with CDI using subjective observations of the extent and number of vessels present within the bowel wall. A normal bowel loop should only show a few color signals within the wall, whereas excess signal is associated with either neoplastic or inflammatory pathology.
Sonographic Technique
Grayscale and Doppler assessment
It is our preference to perform routine bowel examinations after 6 hours of fasting. This shows the bowel in a normal resting physiologic state, avoiding overestimation of vascularity due to a postprandial or functional hyperemia, and avoids fluid-filled loops of peristaltic bowel that may follow a large meal.
Initially, a survey of the bowel is performed using a standard low-frequency convex probe (3–8 MHz). With the patient in a supine position, the probe is placed on the abdomen, and with constant pression, the probe is moved to cover all segments of the bowel using a systematic approach. This technique allows for adequate detection of normal or abnormal bowel segments, assists anatomic localization of an affected loop, and excludes any abnormal deep segments or complications.
There are 2 approaches for bowel scanning that are largely motivated by personal preference of the operator. (1) The most frequently used in the acute setting starts at the right upper quadrant (RUQ) (hepatic flexure of the colon). A downward motion of the probe allows assessment of the ascending colon, cecum, TI, and appendix. This is followed by sequential scanning of the entire colon from the RUQ forward to the level of the rectum. Then, a sweeping motion of the abdomen is used for survey of the small bowel. (2) Starts at the rectum (using the bladder as a sonographic window) and then following the entire colon in a retrograde fashion from the suprapubic region to the right lower quadrant to also included the appendix and TI ( [CR] ). Then a survey of the entire small bowel is performed. This scanning technique is particularly helpful in patients with prior surgeries to assist in the localization of the anastomosis.
After the bowel is surveyed, a higher frequency probe, either curvilinear and/or linear probe (7–18 MHz), is used for a more detailed evaluation of the bowel wall, mesentery, and vascularity. High-frequency probes best delineate the wall layers, detailing transmural disease, serosal abnormalities, sinus tracts, and fistulas. In children and slim individuals, a high-frequency linear probe can be used to perform the entire examination.
If there are localized symptoms in the upper abdomen, a search for the stomach and duodenum is performed. The stomach is generally collapsed in a fasting state and should not show any mural thickening. Some authors suggest oral water ingestion to distend the stomach and upper gastrointestinal (GI) tract to confirm a persistent abnormality. However, ingested air may make this an inconsistent procedure.
All segments of the bowel are imaged using grayscale and Doppler imaging in the axial and longitudinal planes. It is important to optimize Doppler imaging for the detection of low-velocity flow or to use new microcirculation techniques to properly detect mural flow.
Representative images and cine-clips of the affected segment and TI are recorded and stored. Cine-clips are essential for documentation of disease extent, and detection of transition points, subtle mural changes, sinus tracts and fistulas that may have been missed by the operator at the time of the examination. One of the most important contributions of cine-clips is the assessment of bowel motility with identification of normal, excess, or dysfunctional peristalsis. This is essential for the detection of strictures and mechanical bowel obstruction.
Endovaginal scanning
Another important technique in female patients is the addition of endovaginal (EV) scanning, because the cecum, TI, and appendix in some patients are located within the pelvis and can only be seen using an EV approach.
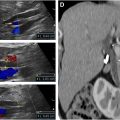
The use of an EV probe allows exceptional visualization of the pelvic loops, and it is sensitive for the detection of abnormal pelvic segments that are not visible by the transabdominal approach ( Fig. 2 A–D and [CR] and [CR] ).

Transperineal scanning
Performed in patients with perianal symptoms, a transperineal scan provides excellent visualization of the anal canal and perianal soft tissues to approximately the level of the anorectal junction. A conventional probe with a plastic cover and abundant gel inside and outside the cover is used. The procedure is initiated in a lithotomy position. A high-frequency linear or curvilinear probe is placed on the perineum between the anal canal and vagina in women and between the scrotum and anus in men. Imaging is performed in the axial and sagittal planes, while maintaining the probe position on the perineum and sweeping from left to right for sagittal and top to bottom for axial imaging. Cine-clips in both planes are recorded.
Ostomy scanning
Ostomy evaluation is also an important component of the bowel assessment. A small footprint probe is placed within the ostomy ring over the bag to visualize the ostomy loop. If this is not possible, the probe then needs to be placed next to the ostomy ring and angle toward the opening until the ostomy loop is well visualized in the field-of-view.
Contrast-enhanced ultrasound
CEUS should be performed on the most abnormal bowel segment, commonly in a long-axis view to facilitate CEUS quantification. Microbubble contrast agents are injected intravenously and a 2 minute videoclip is recorded ( [CR] ). For mural quantification, proprietary software is used to measure the degree of wall enhancement by placing a region of interest (ROI) within the bowel wall. This creates an enhancement curve or time-intensity curve that allows for the determination of peak enhancement (PE) and area under the curve (AUC) as expression of mural vascularity , and disease activity. CEUS is also used for assessment and characterization of inflammatory masses and neoplasms.
Shear wave elastography
Bowel wall stiffness plays an important role in the evaluation of strictures, specially to assist in the management and assessment of treatment response. SWE of the bowel is challenging due to the small size of the target, sonographic compression, bowel motility, adjacent bowel gas, and patient breathing. Nonetheless, investigators have reported optimism for the value of SWE in the assessment of bowel strictures.
SWE is performed using the axial plane of the stricture segment to include as much bowel wall as possible while avoiding lumen and perienteric tissues. An ROI is placed within the abnormal bowel wall, and at least 4 SWE samples are recorded in m/s. Although specific thresholds have not been published, we propose that values greater than 2 m/s will denote significant mural stiffness.
Clinical applications
Acute Abdominal Pain
In the acute setting, US has been commonly used for the evaluation of acute right lower quadrant (RLQ) or left lower quadrant (LLQ) pain. The most common indications include suspected acute appendicitis, diverticulitis, pyloric stenosis, and intussusception, especially in children and young adults. However, other acute intestinal conditions can also be recognized during any sonographic assessment including omental infarcts, mesenteric adenitis with terminal ileitis, acute typhlitis, irritable bowel syndrom (IBD), peptic ulcer disease, and enteritis. Therefore, US evaluation in the acute setting should not be limited to the assessment of the appendix or the area of concern alone. If the etiology is not found with the initial inspection, the sonographic evaluation should be expanded to include the cecum, TI, and a short survey of the small bowel. In patients with diffuse abdominal pain and bloating, a close survey of the bowel is also necessary to detect signs of mechanical bowel obstruction such as dilated segments, dysfunctional peristalsis, or narrowing.
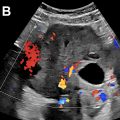
It is known that US has the advantage of having direct input from the patient to localize the area of pain to guide the sonographic assessment. For instance, if pain is localized in the LLQ, the most common explanation is acute diverticulitis ( Fig. 3 A, B ). A diverticulum in US is seen as an outpouching beyond the bowel serosa that can be filled with intestinal content or air appearing as a bright echo with distal shadowing ( Fig. 4 A ). Bacterial overgrowth within the diverticula causes inflammatory changes/acute diverticulitis. This is characterized by hyperemia of the diverticulum and surrounding mesenteric inflammatory changes in the early stage. This can progress to involve a segment of the colon with mural thickening and hyperemia. Localized perforations can occur where echogenic foci representing air are seen adjacent to the inflamed diverticula or colon. An important complication that needs to be recognized is the presence of an abscess ( Fig. 4 B, C). This can appear as a complex fluid collection with peripheral vascularity on CDI or CEUS or as a hypoechoic area within the inflammatory fat. Acute diverticulitis can occur in any part of the intestine; therefore, a careful search of the bowel at the area of pain may help to identify this condition.


Acute appendicitis remains today as the most common cause of the so-called acute abdomen. Images are characterized by demonstration of a dilated appendix, more that 7 to 8 mm in diameter, a fluid-filled lumen with or without appendicolith, periappendiceal inflammatory fat, and hyperemia. Detailed evaluation of the entire appendix, with a focus on the detection of the tip and its wall, is important to identify complications, such as perforation and abscess.
Granulomatous appendicitis is a self-limited process that is treated conservatively. US will nicely demonstrate an increased diameter of the appendix, predominantly related to mural thickening and inflammatory change. The lumen is collapsed a differentiating feature from suppurative appendicitis where the lumen is dilated by fluid/pus most commonly due to an obstructing appendicolith.
Epiploic appendagitis and mesenteric infarcts can also be the cause of localized pain. On US, both are fat-containing echogenic masses and are differentiated only by the location of the mass. Epiploic appendagitis is intimately adjacent to the antimesenteric border of the colon, while mesenteric infarcts are typically located further from the colon.
In children and young adult, intussusception is a common cause of patient’s abdominal pain. This is easily recognized on US by the “target sign” in axial view that represents the invagination of one bowel segment within another. In long axis, it has a “pitch-fork” appearance. In children, intussusception is mostly idiopathic; however, in adults, a close search for a lead point is necessary.
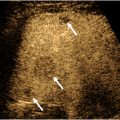
Although much less common today, peptic ulcer disease may be a cause of upper abdominal pain. Ulcers can be recognized as bright echoes with ring-down artifact either within or beyond the wall at the area of pain. Peri-enteric fluid and inflammatory fat can also be seen ( Fig. 5 A, B , [CR] ).