Breast Imaging
Michelle McDonough
Thomas L. Pope Jr.
Jennifer Cranny
The authors and editors acknowledge the contribution of the Chapter 9 authors from the second edition: Lisa F. Baron, MD, and Elizabeth W. Greer and of Dr. Elizabeth DePeri and Dr. Beata Panzegrau from the third edition.
Case 11.1
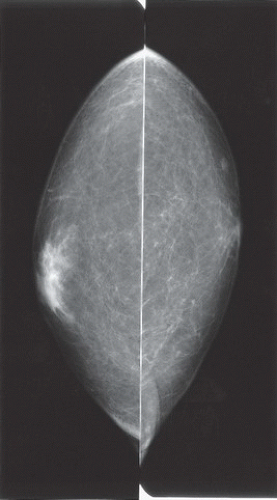
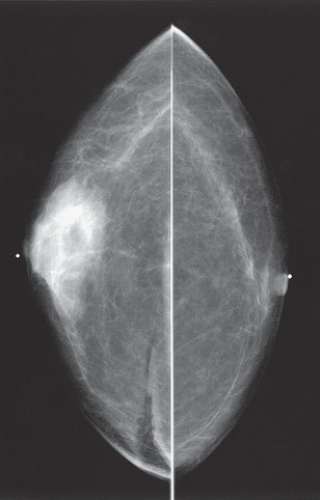
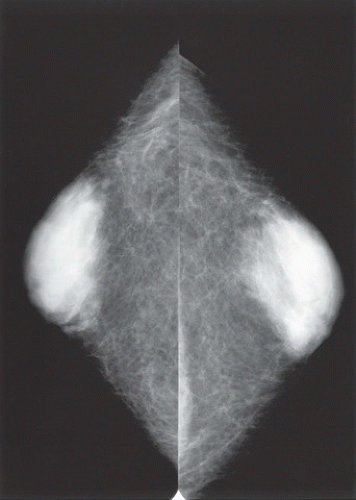
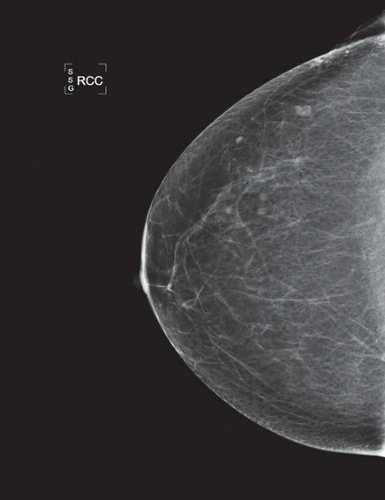
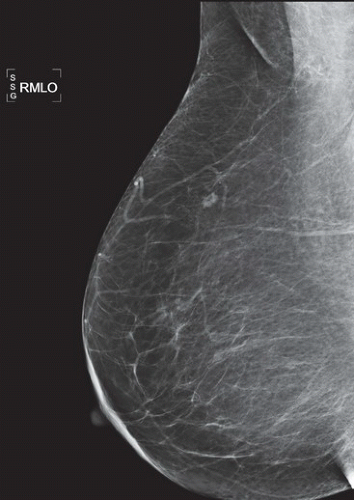
HISTORY: Bilateral craniocaudal (CC) mammograms of three male patients complaining of breast tenderness and fullness
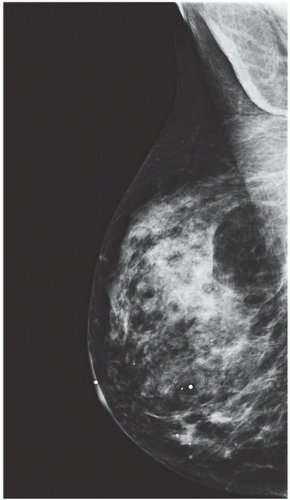
FINDINGS: Bilateral film-screen mammograms demonstrate varying degrees of gynecomastia. Figure 11.1.1 demonstrates mild, asymmetric glandular tissue in a flame-shaped distribution behind the right nipple. The contralateral breast is nearly completely fatty, containing a negligible amount of glandular breast tissue. Figure 11.1.2 depicts a greater degree of asymmetric ductal tissue in the retroareolar area, right greater than left. Figure 11.1.3 illustrates bilateral, symmetric gynecomastia— resembling the normal parenchymal distribution in a female.
DIAGNOSIS: Various mammographic presentations of gynecomastia
DISCUSSION: Gynecomastia is the development of ductal and stromal tissue elements within the male breast without true acinar lobule formation (e.g., fibroadenomas do not occur in men). The causes of gynecomastia can be physiologic, nonphysiologic, or idiopathic. The physiologic form of gynecomastia has a trimodal distribution: neonatal hypertrophy owing to maternal influence of estrogen, pubertal hypertrophy owing to high estradiol levels, and senescent hypertrophy owing to a decline in serum testosterone levels. These peaks are related to a relative increase in estrogen or an overall decrease in testosterone. Underlying disease processes can result in relative estrogen excess and may contribute to the development of gynecomastia: testicular and nontesticular tumors (e.g., lung, liver, adrenal), cirrhosis, nutritional deprivation, androgen deficiency, and systemic disorders. Nonphysiologic causes of gynecomastia include estrogenic active drugs (e.g., anabolic steroids, marijuana, digitalis), drugs that reduce testosterone action or production (e.g., cimetidine, phenytoin, methotrexate,
diazepam), Klinefelter syndrome (e.g., elevated estrogen levels), and ambiguous genitalia syndromes. Nonphysiologic gynecomastia may result from cirrhosis, chronic renal failure, malnutrition, or drugs (1). However, the cause in 40% of these cases is idiopathic (2).
diazepam), Klinefelter syndrome (e.g., elevated estrogen levels), and ambiguous genitalia syndromes. Nonphysiologic gynecomastia may result from cirrhosis, chronic renal failure, malnutrition, or drugs (1). However, the cause in 40% of these cases is idiopathic (2).
The usual clinical presentation of gynecomastia in a man is a complaint of breast tenderness with an associated soft retroareolar tissue mass. The diagnostic workup includes clinical examination and mammography with bilateral CC and mediolateral oblique (MLO) views. Mammography can be useful in differentiating benign from malignant breast disease (3). Gynecomastia has a variable pattern on the mammogram but usually appears as a fan-shaped, triangular density emanating from the nipple. Breast cancer tends to appear as an irregular, eccentric mass with possible calcifications. Ultrasound can be useful in the workup for possible malignancy (4).
Aunt Minnie’s Pearl
Soft, mobile, tender subareolar tissue (unilateral or bilateral) in a man = gynecomastia.
Case 11.2
HISTORY: Withheld
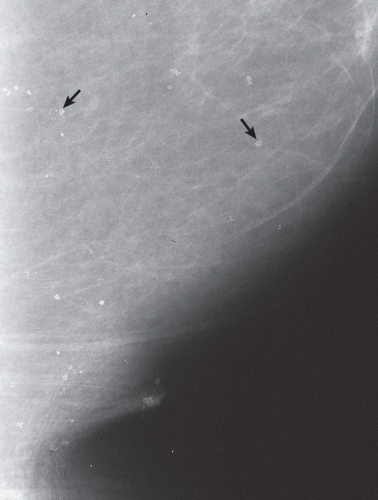
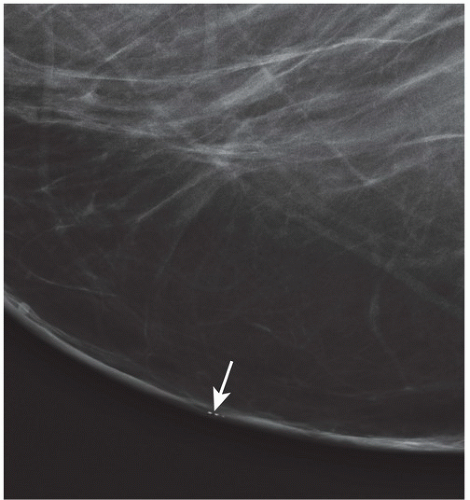
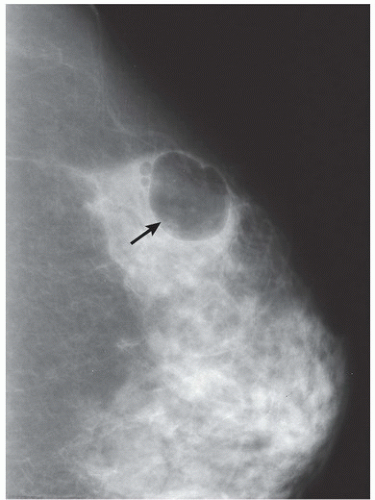
FINDINGS: There are numerous, scattered, ringshaped calcifications (Fig. 11.2.1, arrows) measuring 0.5 to 1 mm in diameter on the CC view of the medial breast. Their superficial location is best appreciated in the cleavage area in this case. The calcifications are mostly single, but some are clustered. Their centers are low density or lucent. The distinguishing feature is the less-than-perfect shape of the calcified ring, which often has a flattened side, giving it a polygonal appearance. Figure 11.2.2 is a supplemental view used to confirm this finding.
DIAGNOSIS: Dermal calcification of sebaceous glands
DISCUSSION: Sebaceous glands occur in the skin in association with hair follicles. When calcified, they are differentiated from fat necrosis by the location in the skin and by their polygonal shape. Their size corresponds to that of skin pores, whereas fat necrosis may have larger rings. Typical dermal calcifications are always benign and do not require biopsy (5). If the classic features are not present, however, the suspected location in the skin can be confirmed with tangential views, thereby avoiding biopsy of atypical skin calcifications that may mimic carcinoma (6).
Aunt Minnie’s Pearls
Polygonal calcified rings in a superficial location = dermal calcifications.
Tangential views confirm the diagnosis.
Case 11.3
HISTORY: Palpable finding in medial left breast
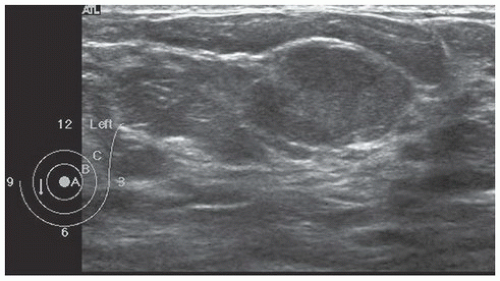
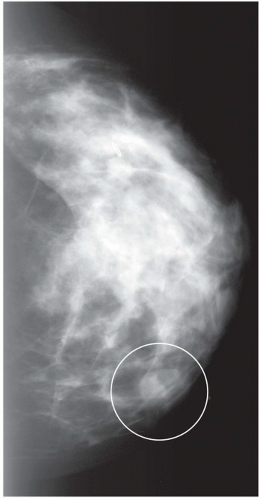
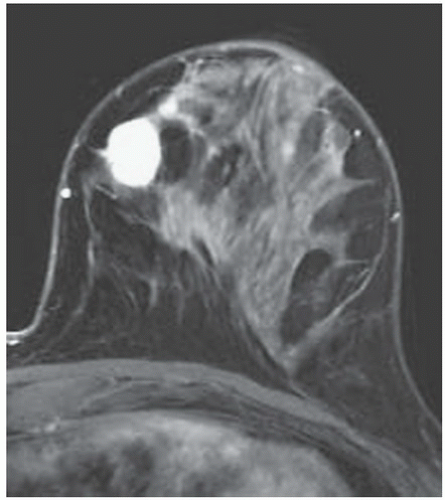
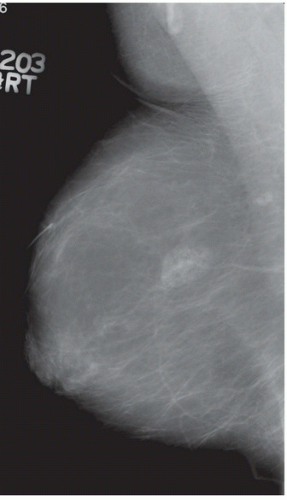
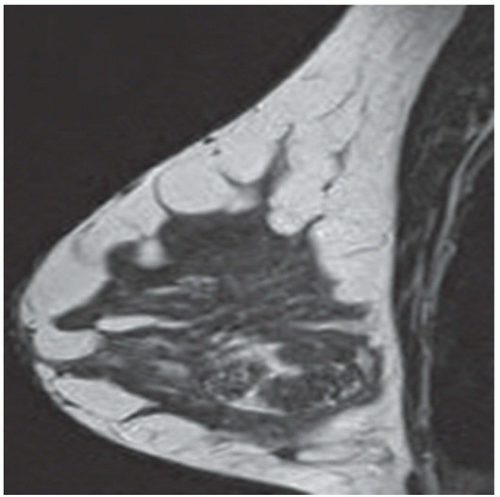
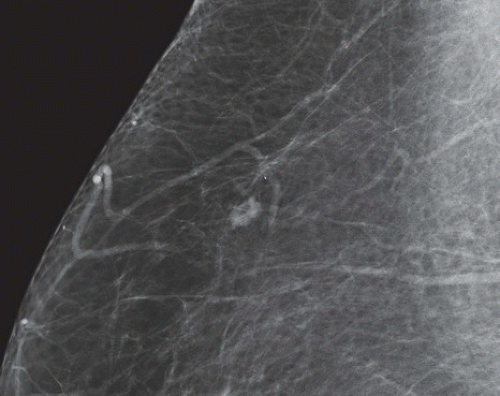
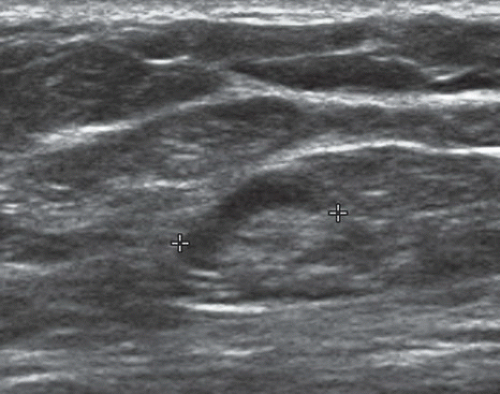
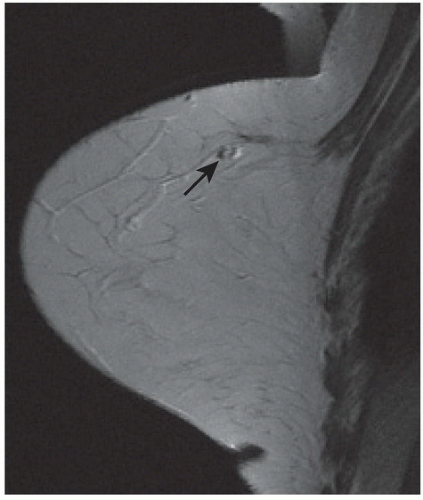
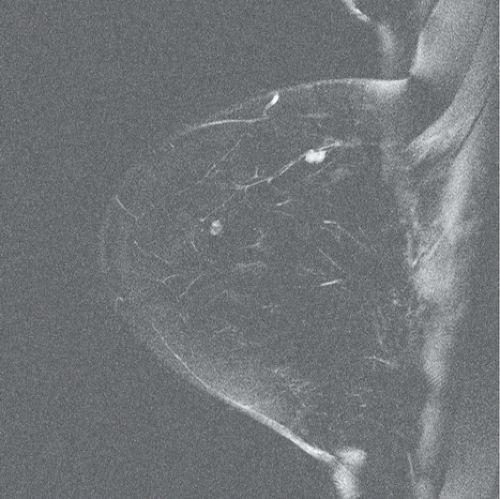
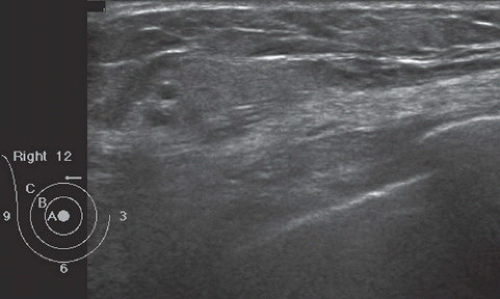
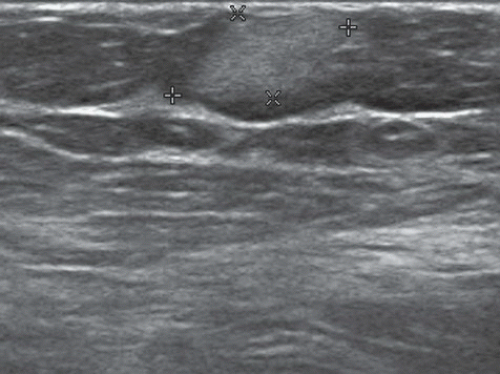
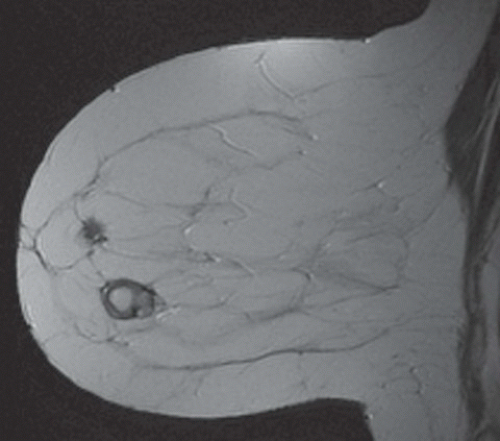
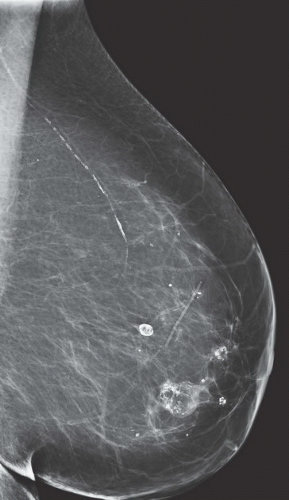
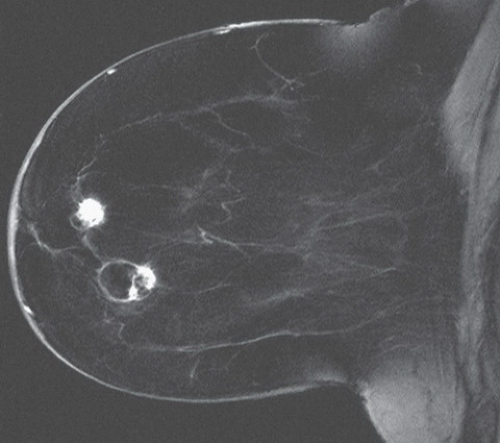
FINDINGS: Partially circumscribed mass medial left breast on mammography CC view (circled Fig 11.3.1) and incidental note of radiopaque marker in lateral aspect of breast. Ultrasound (Fig. 11.3.2) demonstrates a circumscribed mass with macrolobulations, mild internal heterogeneity, and acoustic enhancement correlating with the mammogram and palpable concern. A medial, circumscribed, ovoid mass intensely enhances on gadolinium-enhanced axial T1-weighted MR image (Fig. 11.3.3), on MR examination performed for contralateral breast concern.
DIAGNOSIS: Fibroadenoma
DISCUSSION: A fibroadenoma is a common benign tumor, occurring frequently in young women. Their growth is stimulated by estrogen, and peak incidence is between the ages of 25 and 35 years, decreasing around 40 years. The juvenile fibroadenoma occurs during puberty and has a tendency to grow to larger sizes. Therefore, complete surgical excision may be indicated. The adult variety typically occurs in young women and is usually smooth, rubbery, and clinically mobile and cannot be reliably distinguished from a circumscribed carcinoma (such as medullary or colloid/mucinous carcinoma) on the first mammogram. The absence of growth
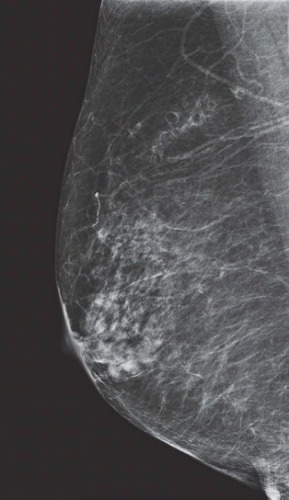
over several years is typical although some fibroadenomas increase in size over time. With advancing age and during menopause, the fibroadenoma usually degenerates with a decrease in the size of the mass while showing an increase in coarse, popcornlike calcifications (Fig. 11.3.4 shows classic fibroadenoma course calcifications (7).
over several years is typical although some fibroadenomas increase in size over time. With advancing age and during menopause, the fibroadenoma usually degenerates with a decrease in the size of the mass while showing an increase in coarse, popcornlike calcifications (Fig. 11.3.4 shows classic fibroadenoma course calcifications (7).
Fibroadenomas are classically ovoid or almondshaped with smooth borders, internal homogeneous echogenicity, and acoustic enhancement. They can be incidental or evaluated because they are palpable. Multiplicity is often seen. In the majority of patients, in particular if there are any unusual features present, histologic confirmation of fibroadenoma with postbiopsy clip placement is chosen. Some clinicians will choose short-term follow-up imaging in young patients with typical findings of fibroadenoma because the risk of malignant transformation or associated malignancy is extremely low (8). Finally, some patients may prefer to have the lesion removed; ultrasound-guided percutaneous excision or cryoablation are two minimally invasive techniques to remove this benign tumor (9,10).
Aunt Minnie’s Pearls
Classic-appearing fibroadenomas can fluctuate slightly in size, by some reports up to 20% in volume, without concern. If they are atypical in appearance, imaging characteristics change, or they grow more rapidly, tissue diagnosis should be considered (11).
If palpable, they can then be followed up clinically. If not palpable, they can be followed with imaging. A reasonable plan is follow-up at 6-month intervals for 1 year and then annual imaging after size stability is established. If the patient is younger than 40 years and would not otherwise be imaged annually, excision is a reasonable option to eliminate the need for annual exams.
Some surgeons will remove fibroadenomas for large size rather than clinical or imaging follow-up. The size criteria for excision will vary, ranging from 20 to 30 mm.
Case 11.4
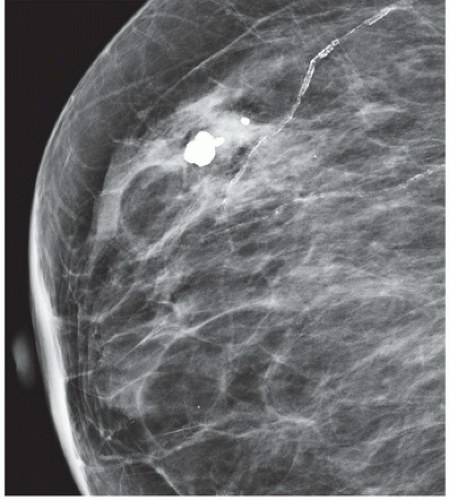
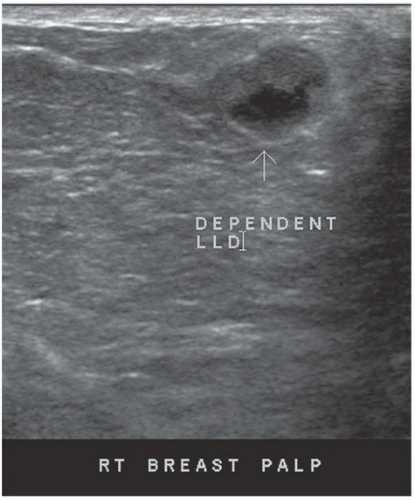
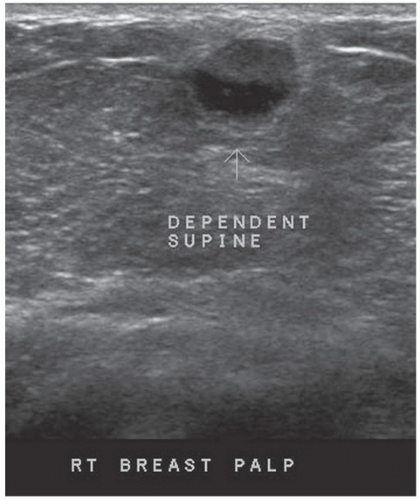
HISTORY: A 34-year-old lactating woman 4 months postpartum with a palpable nontender lump
FINDINGS: Complex cystic mass with positiondependent eccentric echogenicity (Figs. 11.4.1 and 11.4.2).
DIAGNOSIS: Galactocele
DISCUSSION: A galactocele is a milk- or fat-containing cystic mass that results from cystic dilation of a duct during or after lactation. It occurs in women of childbearing age and is often palpable. It is most commonly nontender. The galactocele is diagnosed by visualization of a fat-fluid level, either on upright lateral mammogram or ultrasound, where they are usually well-circumscribed (12). All fat-containing masses in the breast are benign; therefore, the identification of fat within the mass may eliminate the need for definitive diagnosis. If ambiguous imaging characteristics are present, such as acoustic shadowing, aspiration might be considered for diagnosis. Within the aspirate, a fat-fluid level may be visible within the syringe, or the fat component of the aspirate may float if the aspirate is added to a liquid phase fixative (13).
Aunt Minnie’s Pearls
If a lesion is thought to represent a galactocele, image the mass sonographically with the patient in different positions to demonstrate the mobility of the fat-containing portion of the mass and to confirm a fat-fluid level that makes the diagnosis.
Any fat-containing breast mass is benign.
Case 11.5
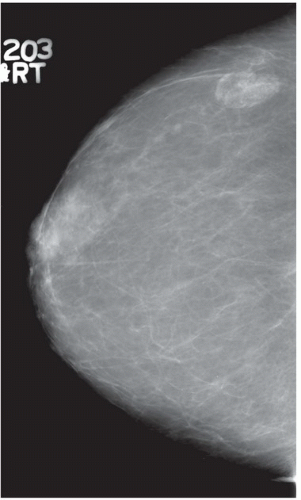
HISTORY: Right breast mammogram obtained in a 63-year-old woman during annual examination
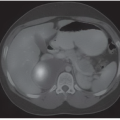
FINDINGS: Unilateral right breast film screen mammogram in CC and MLO projections (Figs. 11.5.1 and 11.5.2). Images show a 2-cm mixed density fat containing ovoid mass in the upper outer quadrant. T2 nonfat suppressed sagittal MRI (Fig. 11.5.3) shows a well-circumscribed 4.5-cm mass with visible fat and glandular elements contained within a visible capsule.
DIAGNOSIS: Hamartoma (fibroadenolipoma)
DISCUSSION: Hamartomas, also known as fibroadenolipomas, are a benign proliferation of fibrous, glandular, and fatty tissue elements surrounded by a thin pseudocapsule of connective tissue (11). On mammography, these masses have a typical round to oval configuration and contain visible soft-tissue density and fatty elements. The appearance of the fatty internal elements indicates a benign lesion that requires no additional workup or biopsy. These lesions may be palpable on physical examination. They are typically soft and freely mobile. Size can vary considerably from a few millimeters to several centimeters (12).
Risk for developing breast cancer is the same as the surrounding breast tissue and therefore is not increased over baseline. However, breast cancer can develop in any location that contains glandular elements, so assessment for interval change is always warranted (13).
Diagnosis is typically made by mammography alone and ultrasound is not usually required owing to its classic appearance. They can be found inadvertently on MRI with expected T1 and T2 elements of fat, fibrous tissue, and a pseudocapsule (14).
Aunt Minnie’s Pearls
Include in the differential diagnosis of fat-containing masses on mammography along with lymph nodes, lipomas, fat necrosis, and galactoceles.
These masses are almost always benign; however, as they do contain glandular elements, evaluation for interval growth or suspicious change is required.
Case 11.6
HISTORY: A 50-year-old woman with a palpable mass in the upper outer quadrant of the left breast
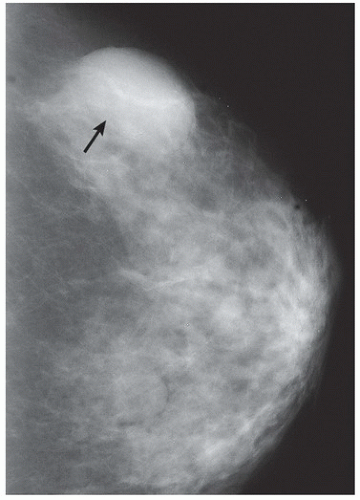
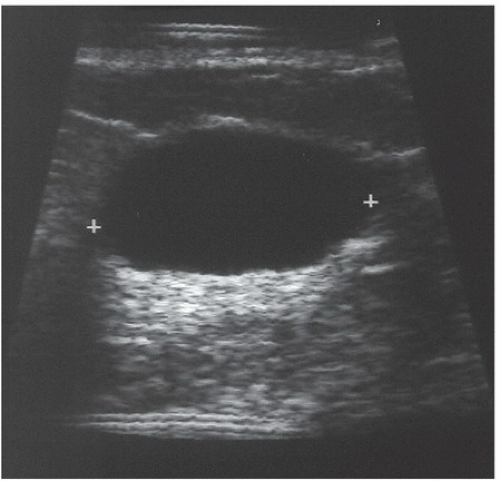
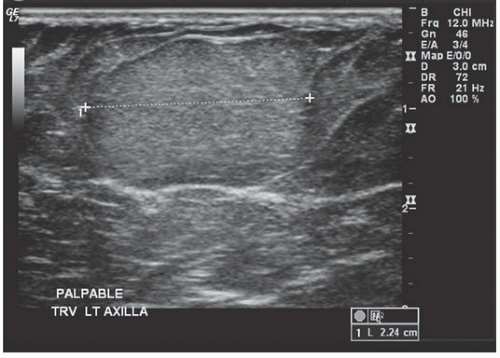
FINDINGS: A CC view of the left breast (Fig. 11.6.1) demonstrates a 3.5-cm oval mass located in the lateral portion of the breast with well-circumscribed margins (arrow). Breast tissue is visible “through” the lesion, indicating that it is a low-density, radiopaque mass. Sonography (Fig. 11.6.2) shows a completely anechoic, smooth-walled structure with a sharp back wall and enhanced through-transmission (between cursors). A postultrasound aspiration pneumocystogram (Fig. 11.6.3) reveals a well-demarcated, air-filled structure with a smooth inner wall (arrow).
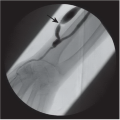
DIAGNOSIS: Simple breast cyst
DISCUSSION: Mammograms do not detect breast cysts; instead, they show a mass that requires characterization with ultrasound. Ultrasonography classifies a mammographic lesion as a simple cyst when the mass has the following characteristics: smooth thin wall, completely anechoic pattern, and distal acoustic enhancement. Complicated cysts have a benign appearance but may contain a few internal echoes. This is likely owing to improved ultrasound technology. These can often be considered benign if there are no other concerning features. Complex cysts are lesions that lack the characteristics of a simple cyst and may contain one or more of the following features: irregular or thick wall, internal echoes, and diminished acoustic enhancement. Percutaneous aspiration or biopsy of complex cysts is frequently recommended.
Patients may present with a palpable mass, prompting the clinician to request mammography. In general, the definitive diagnosis requires both mammography and ultrasonography. Only when all criteria exhibited in Figure 11.6.2 are met can a simple cyst be diagnosed with confidence. Historically, pneumocystography could be performed to demonstrate a smooth inner wall by using air as the contrast medium. This procedure was performed mainly to exclude an intracystic mass and to correlate with mammographic findings (15,16).
Aunt Minnie’s Pearls
Ultrasound findings of smooth thin wall, anechoic signal, and distal acoustic enhancement = benign breast cyst.
Complex cysts should be aspirated and/or biopsied if they are not typical of a simple cyst.
Case 11.7
HISTORY: Mammogram and ultrasound images from two patients with the same diagnosis
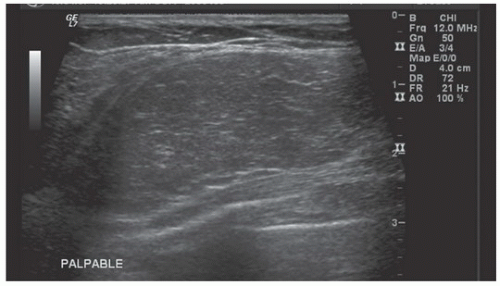
FINDINGS: MLO view of the right breast (Fig. 11.7.1) demonstrates a large, well-circumscribed, lucent lesion with thin capsule in the upper breast. Ultrasound image (Fig. 11.7.2) demonstrates well-circumscribed, homogeneous, hyperechoic lesion corresponding to mammographic abnormality. The second image figure (Fig. 11.7.3) demonstrates well-circumscribed, homogeneous, hypoechoic lesion in a different patient with the same pathology.
DIAGNOSIS: Lipoma
DISCUSSION: The lipoma is a common, asymptomatic, benign breast lesion, which is typically radiolucent on mammographic imaging. It is usually superficially located and has well-defined borders with very thin capsule. It may distort surrounding tissue by displacing adjacent breast parenchyma. When necrosis occurs, calcifications may be present. On physical examination, lipomas are typically soft, freely movable masses (17). Sonographic appearance may vary from hypoechoic to hyperechoic, but well-circumscribed margins and homogenous appearance are typical.
Aunt Minnie’s Pearl
Radiolucent lesion with thin capsule is diagnostic of lipoma.
Case 11.8
HISTORY: Images of the right breast in a woman with prior history of benign breast biopsy
FINDINGS: Mammographic images in the CC and MLO projections (Figs. 11.8.1 and 11.8.2 and pictographic magnification Fig. 11.8.3) show a 10-mm ovoid mass in the upper outer quadrant with a fatty hilum. Ultrasound examination confirms a mass with central increased echogenicity consistent with a fatty hilum and a hypoechoic but thin cortex (Fig. 11.8.4). Sagittal T2-weighted MRI image without fat saturation shows the same ovoid mass with peripheral low-signal intensity and central fat signal adjacent to a blood vessel with visible chemical shift artifact (Fig. 11.8.5, arrow). Same sagittal MRI slice with T1-weighted imaging, fat saturation, and contrast. The ovoid mass shows fatty notch of the central aspect with enhancement of the cortical rim (Fig. 11.8.6).
DIAGNOSIS: Intramammary lymph node
DISCUSSION: Intramammary lymph nodes are commonly found in the upper outer quadrant and axillary tail of the breast but can be found anywhere in the breast. They can vary in size but are typically ovoid or reniform in shape. The single best distinguishing feature on mammogram is a notch or central area of radiolucency corresponding to the fatty hilum. If this finding can be identified with certainty, additional imaging can be avoided (18). However, if a fatty hilum cannot be established at mammogram, ultrasound may be performed for further evaluation. Ultrasound can be used as an adjunct to mammography or as a primary method of evaluation in patients under the age of 30. At ultrasound, the mass is well-circumscribed with a peripheral hypoechoic rim or cortex and a central area of increased echogenicity corresponding to the fatty hilus. Blood flow to the central echogenic aspect of the mass can often be established with color Doppler exam (19). MR imaging is not typically done to evaluate lymph nodes, but they may often be seen as incidental findings for other indications. On T2-weighted images without fat saturation, the fatty hilum with its central high-signal intensity can often be established. Peripheral cortical rim is of low-signal intensity. On T1-weighted post-contrast imaging, the peripheral cortical rim will often enhance. If fat saturation has been used to improve lesion conspicuity, the central fatty hilus will “disappear,” will be suppressed. Lymph nodes can often be found immediately adjacent to a feeding vessel. Although mammogram, ultrasound, and MRI cannot determine metastatic disease within lymph nodes, certain features can suggest an increased likelihood of malignant involvement including increased size and density of the node on mammography, loss of the fatty hilum, increased cortical thickness, ill-defined or spiculated margins, and intranodal microcalcifications (20).
Aunt Minnie’s Pearls
Well-circumscribed reniform mass with central radiolucency, “notch,” often in the upper outer quadrant/axillary tail of the breast. Ultarsound and MRI may show a central feeding vessel.
Suspect metastatic involvement if there is increased size and density, loss of the fatty hilum, or irregular margins.
Case 11.9
HISTORY: Several cases are shown; histories are withheld
FINDINGS: Mammogram mediolateral (ML) view demonstrates an elongated mass superiorly with several rounded lucencies (Fig. 11.9.1). Ultrasound of the corresponding area shows increased echogenicity masses with cystic components (Fig. 11.9.2). In a different patient, ultrasound demonstrates a uniformly hyperechoic superficial mass (Fig. 11.9.3). A third patient has a fat-density mass with rim calcifications, sometimes called eggshell calcifications, in the anterior breast, shown here on an MLO view (Fig. 11.9.4). Corresponding MR images with T2-weighting (Fig. 11.9.5) and T1-weighting (Fig. 11.9.6) with contrast demonstrate fat signal-containing masses with a thin enhancing anterior wall and slight irregularity with enhancement posteriorly as well as a second low T2-enhancing irregular mass superiorly in the anterior breast, which corresponds well to dystrophic calcifications on the mammogram.
DIAGNOSIS: Fat necrosis
DISCUSSION: Fat necrosis has many appearances and can be a mimicker of tumor on all imaging modalities. A fat-containing mass with eggshell calcifications is a classic description for fat necrosis (21). However, depending on the age of the process and the inflammatory reaction that it incites, the findings can vary and include spiculated masses and irregular enhancing masses on MR, where fat signal can sometimes be obscured. Ultrasound typically shows hyperechoic masses, but in a more inflammatory state, findings can be more equivocal, with ill-defined masses of lower echogenicity.
Aunt Minnie’s Pearls
Fat necrosis can be a mimicker of tumor. Without definitive findings and correlative history, biopsy should be considered (22).
MR is helpful to identify fat within a mass, particularly in a scar where overlying density makes fat less apparent on mammography or fibrosis within the scar shadows on ultrasound.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree