Breast Ultrasound
Ultrasound is a technology that seems to improve each year, but it still has important limitations. Those who believe that ultrasound can provide nearly histologic detail that goes beyond what has been shown scientifically will likely be disappointed with this chapter. As in all aspects of health care, the scientific evidence should take precedent over anecdote. I have been using ultrasound to evaluate the breast since the late 1970s. It has become an increasingly useful tool in breast evaluation. My approach, however, is a practical one. For example, I have little doubt that, on rare occasions, what I am seeing with ultrasound is indeed “ductal extension” (breast cancer filling a duct adjacent to the primary tumor). However, rather than using this rare event to suggest that it is something that can be seen routinely, I would classify it as an unusual occurrence that is unconfirmed and of uncertain importance. I would challenge those who feel such findings are easily seen and of some consequence to do the appropriate studies to prove their contentions. I have seen signficant errors made by those who have tried to read more into the ultrasound study than can legitimately be deduced. Unsubstantiated speculation using an imaging technique is fine as long as it does not affect patient care.
Breast ultrasound could benefit from a more scientific approach. I will try in this chapter to provide the science when it is available and to indicate whenever possible that the conclusion is anecdotal and not based on science.
Despite the marked improvements in technology and the vast improvements in image quality that have occurred over the past decade, ultrasound remains primarily a method for differentiating cystic lesions from solid masses and for guiding interventional procedures (aspiration, localization, and core biopsies). As with any test, there is great interest in using sonography diagnostically to go beyond cyst/solid differentiation, and some believe they can use ultrasound to distinguish some benign solid masses from malignant masses. This will be discussed below.
An additional controversial area is the use of ultrasound to screen for breast cancer. Although older studies were unable to demonstrate any efficacy for ultrasound as a screening technique (1), several more recent reports suggest that improvements in ultrasound technology may permit ultrasound to detect some cancers that are occult to clinical and mammographic evaluation (2). Those who argue for using ultrasound for breast cancer screening, however, have not understood the lessons from the mammography debates of the past and the important issues that are involved in the validation of a screening test (3). Because screening is used to evaluate healthy individuals, the false-positive findings associated with it have some potentially negative consequences. As a result, screening requires a different level of validation than a test that is applied to someone who is already ill (see Ultrasound Screening for Breast Cancer). One critical question that has not yet been answered about screening for breast cancer using ultrasound is whether or not finding cancers that are evident only by ultrasound actually results in saving lives.
The following discussions take a very practical approach to the use of ultrasound for breast evaluation. I do not mean in any way to discount the apparent ability of some to see what others cannot with this highly operator-dependent technology, but rather to provide a practical overview that can be applied by most who use ultrasound in breast evaluation.
Ultrasound of the Breast
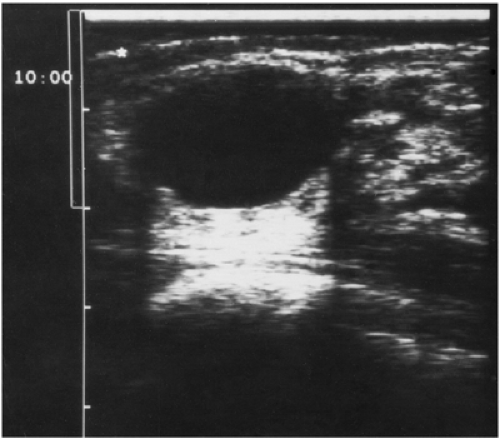
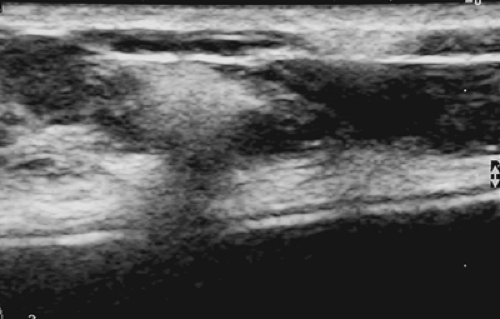
The actual details of ultrasound technology seem to change on an almost annual basis, so it is impossible to scientifically evaluate every new technologic change. The practitioner must rely on information from the manufacturers for details of each new modification. I would also urge that when purchasing a new ultrasound device, there should be a trial period so that the practitioner can evaluate for himself or herself the image quality. The two most important requirements for breast ultrsound are that the device be able to accurately differentiate a cyst from a solid lesion, and a needle should be clearly visible in breast tissue so that the ultrasound device can be used to guide needle aspiration and biopsy procedures. Cysts should ideally not have any internal echoes, and they should have sharply defined margins. Most fibroadenomas should also have sharply defined margins. Stavros has suggested that solid benign lesions
should have a thin, echogenic pseudocapsule (Fig. 17-1). Although this finding is far from universal, if the practitioner plans to use this detail, the sonographic device should be capable of delineating it.
should have a thin, echogenic pseudocapsule (Fig. 17-1). Although this finding is far from universal, if the practitioner plans to use this detail, the sonographic device should be capable of delineating it.
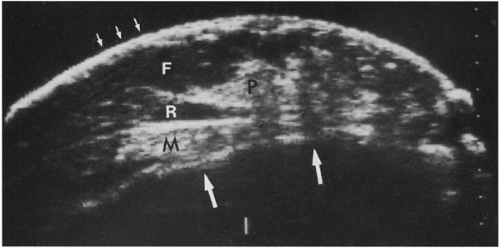
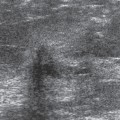
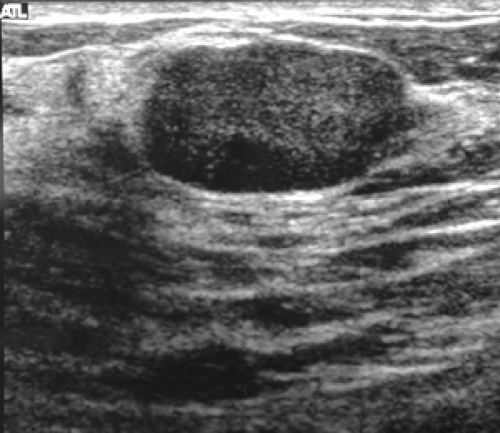 Figure 17-1 This benign fibroadenoma demonstrates a thin, echogenic “pseudocapsule” defining its margin. |
Sonography of the breast depends on the production of high-frequency sound waves that penetrate into and through the tissues. These pass through tissue, are scattered off in directions away from the transducer, are absorbed by the tissues, or are reflected back to the transducer. The process can be thought of simplistically. A pulse of sound is produced by the transducer and a clock starts. The sound enters the breast as a wave, and as it passes through the tissues some of the sound reflects back to the transducer, which is now in the “listening” mode. By timing how long the sound takes from when it was generated, bounced back, and when it was “heard” by the transducer, and by knowing approximately how long it takes for sound to travel through breast tissue, the computer can place a mark on the monitor that corresponds to the location (depth) of the structure that had reflected the sound back to the transducer. Again, as an oversimplification, the sound is generated and covers x centimeters in y seconds (the speed of sound in breast tissue). It travels down to the reflector and bounces back to the transducer; since it passes through the same tissues, the return trip takes approximately the same amount of time back as it took to go down, and the amount of time it takes to make the round trip can be divided by two to calculate how far away from the transducer the reflection occurred. The computer can then paint a spot on the monitor whose location has been calculated as described above and whose brightness is related to the strength of the signal that bounces back to the transducer. The computer registers the reflections coming back in this way from sound sent numerous times per second so that a “real-time” image of the tissues can be seen.
The sound waves are not just reflected; much of the sound is scattered by the tissues so that it does not bounce back toward the transducer but is deflected off in other directions. The tissues also absorb the sound, converting it to heat (other forms of ultrasound can be used specifically to heat tissues as a therapeutic intervention). Because the sound beam is scattered and attenuated as it passes through the tissues, the strength of the signal that bounces back to the transducer is weakened rapidly with increasing depth penetration. The device’s computer can be used to correct for this by boosting the apparent signal decrease to compensate for loss with increasing depth, providing a more uniform and pleasing image for interpretation.
Ultrasound has numerous other characteristics that must be taken into account in an effort to produce high-quality images. The transducer face pulses out waves of sound that parallel its surface. The structures struck directly by these waves are seen most clearly. Since this is in the axis of the waves, the resolving power in this direction is called the axial resolution. The ability of the beam to resolve structures along the side of the beam is not as good (lateral resolution). Furthermore, since sound waves are bent by the tissue through which they pass, the beam begins to spread with increasing depth, causing a “defocusing.” The manufacturers have developed varying methods for compensating for these phenomena, including better methods for focusing the sound and dealing with other forms of image degradation. The reader must decide for himself or herself whether the various methods of signal processing provided by the vendors enhance the quality of the ultrasound image.
With the exception of some experimental devices, breast ultrasound is primarily performed using hand-held, real-time systems. With rare exception, high-frequency transducers should be used. Generally 7.5-MHz systems or higher are needed to provide the resolution needed for breast imaging. Since many breasts can be thinned to only a few centimeters with the patient supine, depth penetration is not as important as in other parts of the body, and high frequencies (12 MHz and up) can be used. It remains to be seen how high frequencies can go, since some of their energy gets deposited in the form of tissue heating. Although higher frequency generally means higher spatial resolution, the heat and possible cavitation of higher-frequency systems may cause tissue damage and preclude their use. Transducers used for breast evaluation should be focused to the near field to permit high resolution from the skin to a depth of 4 to 5 cm.
Although trained technologists can perform breast ultrasound, we believe that the study is best performed by a radiologist trained in breast imaging. The ultrasound findings are frequently correlated with those on the mammogram or other breast imaging study, such as magnetic resonance imaging (MRI), so a physician skilled in both studies
is preferred. There are many normal structures in the breast that can be mistaken for pathology, and benign cysts are common. It has been my experience that technologists frequently find and focus on a cyst that actually proves to be coincidental rather than on the more subtle pathology. I believe that the radiologist is best suited for differentiating significant findings from others.
is preferred. There are many normal structures in the breast that can be mistaken for pathology, and benign cysts are common. It has been my experience that technologists frequently find and focus on a cyst that actually proves to be coincidental rather than on the more subtle pathology. I believe that the radiologist is best suited for differentiating significant findings from others.
Anatomy of The Breast As Delineated by Ultrasound
Ultrasound can demonstrate the skin, subcutaneous fat, breast parenchyma, retromammary fat, pectoralis muscle, ribs, and anterior chest wall (Fig. 17-2). Ducts are frequently visible beneath the nipple, where they are normally somewhat larger (lactiferous sinuses) than those deeper in the breast and where duct ectasia is most common. Fat is the most ubiquitous and obvious tissue in the breast. Since there are no real anatomic landmarks in the breast other than the nipple/areolar complex, image adjustment and lesion analysis are related to the echo characteristics of the breast fat. One unusual characteristic of the breast tissues that differs from other parts of the body is that relative to the parenchyma, fat in the breast appears hypoechoic. This has never been studied in great detail. It is not clear whether the fat of the breast actaully differs from fat in other parts of the body or whether this is a phenomenon of relative echogenicities. I favor a combination. I suspect that the tissues that support fat in the breast may differ from those tissues elsewhere so that there are fewer reflecting surfaces within breast fat. Since the fibroglandular structures of the breast are quite echogenic, the fat is relatively less echogenic in comparison. I believe these combine to produce the relatively hypoechogenic appearance of fat in the breast.
The fact that fat in the breast is hypoechoic poses some problems for sonographic analysis. With rare exceptions, breast masses and in particular breast cancers are also hypoechoic. Fortunately the hypoechogenicity of most cancers can be seen against the background echotecture of the fibroglandular breast tissue, but a significant number of breast cancers are difficult if not impossible to see using ultrasound because they are isoechoic with the fat of the breast tissue.
Ultrasound for Breast Cancer Screening
Early History of Ultrasound as a Screening Technique
In the 1970s and early 1980s, as a consequence of the exaggerated concern over mammography and the overestimated radiation risk to the breast, investigators looked to ultrasound as a replacement for mammographic screening. To facilitate ultrasound screening, whole-breast ultrasound units were devised that permitted scanning of the entire breast to try to detect early-stage breast cancer. Several large studies showed that ultrasound, at that time, was incapable of detecting cancers that were not also evident by either physical examination or mammography (1,4). As noted above, however, some investigators, using more modern equipment, have reported cancers detected only by ultrasound. Although encouraging, these studies have not been properly performed (blinded interpretation), and the scan techniques and criteria for intervention were not clearly defined. Although ultrasound is expected to be used in conjunction with mammography screening, the fact that it is being used to try to find unsuspected cancer makes it a true screening test, and it must be properly validated. To truly understand the ability of a secondary screening test to find cancer, it must first be studied in a blinded fashion so that the interpreter of the ultrasound examination does not know the results of the mammogram or clinical breast examination. Once the blinded evaluation is completed, then it can be reinterpreted with the information from the other studies. Without blinding, the ultrasound study can be biased by the information from the other studies. Without blinding, a divining rod could be shown to point to breast
cancer (see Ultrasound Screening for Breast Cancer). None of the studies to date has been blinded. The ACRIN/Avon trial 6666 is a blinded study and will be the first to determine the true ability of ultrasound to find mammographically occult cancers.
cancer (see Ultrasound Screening for Breast Cancer). None of the studies to date has been blinded. The ACRIN/Avon trial 6666 is a blinded study and will be the first to determine the true ability of ultrasound to find mammographically occult cancers.
The biggest hurdle for a breast cancer screening test, however, is to prove that finding cancers using the new test actually results in a decrease in cancer deaths. Without this proof, a screening test may not only not be efficacious, but, because of false-positive studies, its use could actually be detrimental.
Although we must wait for the proper studies and scientific proof of benefit, I think that the mounting evidence suggests that ultrasound may be able to detect some early cancers that are occult on mammography and clinical breast examination. If this proves to be the case, systems for whole-breast ultrasound evaluation will need to be developed to remove the current operator dependence of the procedure. High-quality mammography remains the only proven imaging technique that has efficacy for the detection of clinically occult early-stage cancer, but as technology improves other tests may begin to show efficacy.
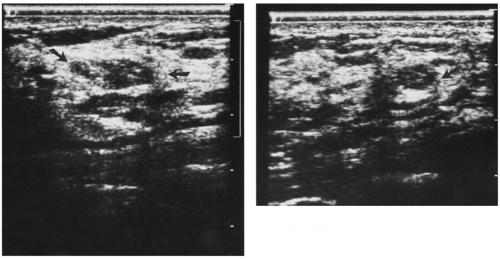
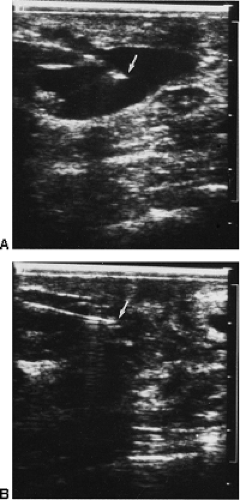
In our experience with hand-held, real-time contact breast ultrasound, as well as our original experience with whole-breast scanning with a dedicated water-path system, we frequently have had difficulty separating the hypoechoic, ill-defined appearance of breast cancer from normal, irregularly marginated, hypoechoic mammary tissue (Fig. 17-3). The normal structures of the breast produce findings on ultrasound that are similar to those seen with some breast cancers (Fig. 17-4). Fat is hypoechoic, and “fibrocystic” tissue can mimic the hypoechogenicity of breast cancer. Cooper’s ligaments and other fibrous structures in the breast can produce acoustic shadowing that can also
be indistinguishable from breast cancer (Fig. 17-5). If these problems can be overcome so that the false-positive rate for ultrasound becomes reasonable, and if ultrasound can be shown to have screening efficacy (decreases deaths) in properly performed trials, it could be a second-level screen, in addition to mammography, for women with dense breast tissue.
be indistinguishable from breast cancer (Fig. 17-5). If these problems can be overcome so that the false-positive rate for ultrasound becomes reasonable, and if ultrasound can be shown to have screening efficacy (decreases deaths) in properly performed trials, it could be a second-level screen, in addition to mammography, for women with dense breast tissue.
The fact that ultrasound finds lesions that mimic breast cancers but prove to be benign (false-positives) was originally demonstrated in our study of more than 1,000 women. We undertook long-term follow-up of 94 women who had suspicious lesions found only by ultrasound (they all had normal clinical breast examinations and mammograms). Over a 3- to 4-year period, none of these women developed a clinically evident cancer. This study demonstrated that scanning women with normal mammograms and negative physical examinations raises concern for lesions that are invariably benign. There is as yet no support for surveying the entire breast by ultrasound, and with a high false-positive rate, scanning the breast is undesirable unless it is part of a research protocol. Our study used old, whole-breast ultrasound imaging, but it provides a model for how the ability of ultrasound to find cancers should be tested. It was prospective and the readers were blinded. The radiologist who interpreted the mammograms did not know the results of the ultrasound or the clinical examination. The radiologist who interpreted the ultrasound study (performed in a standardized, automated fashion) was blinded to the findings on the clinical examination and the mammogram. This is the only way to determine whether there is a separate, efficacious role for a test. Despite anecdotal claims, there has not yet been a prospective, blinded study of lesions detected only by ultrasound that validates its use for detecting clinically and mammographically occult breast cancer in asymptomatic women. These observations do not negate the usefulness of ultrasound in diagnosis for evaluating lesions whose presence has been established by physical examination or mammography.
Support for Screening for Breast Cancer Using Ultrasound
Investigators have described the detection of cancer by ultrasound alone, but these reports remain anecdotal (1,5,6,7,8,9,10,11,12). The selection criteria in many have not been clearly defined, and the scan techniques as well as criteria for intervention have not been clearly documented. One of the biggest problem with interpreting the results from these studies lies in the fact that the investigators using ultrasound to “detect” breast cancers have not been blinded to the clinical examination and the mammogram (13). What all have failed to understand is that the only way to prevent bias would be to conduct blinded interpretation of the ultrasound without access to the mammogram and clinical examination. The reason that this is so important is that blinded analysis is the only way to determine the independent contribution of a new technology for screening. If the individual evaluating the new technology (in this case ultrasound) has access to other information, that information can bias the evaluation. Consciously or otherwise, the analyst may use the information from the mammogram or the clinical examination to “detect” an abnormality using ultrasound that would not have been detected without that information. It has been argued that ultrasound would never be used without mammography for screening, but this is irrelevant. Without knowing the independent contribution of ultrasound, its use in conjunction with mammography is indeterminate. Once the blinded comparision is made, then the analyst can have access to all of the data, and the analysis can determine how this changes from the independent review (14). This is the only way to determine how ultrasound functions as a screening test.
Even after the sensitivity of ultrasound for detecting occult cancer is determined, it remains to be seen whether finding cancers by ultrasound alone actually saves lives. The same issues involved in demonstrating whether mammography screening is efficacious must be addressed for ultrasound screening.
The reason validation is so important can be summarized in the following manner. Suppose that ultrasound screening found only the cancers that were nonlethal (most women who develop breast cancer do not die from it). On the other hand, what if ultrasound found only cancers that had already metastasized and were incurable even though they were found earlier? If either or both of these situations is the case, then finding cancer using ultrasound would be of no benefit. Since ultrasound has a very high false-positive rate and false-positive results lead to biopsies, and since every cancer that is detected will be treated, a screening test that finds only incurable or nonlethal cancers will cause
only harm, with no benefit. It is for this reason that rigorous testing is needed to prove that a screening test has efficacy (15). It is clear that there are palpable cancers that are not visible by mammography but are visible by ultrasound (16). What is not clear is how many cancers that are not palpable and not visible by mammography are detectable by ultrasound.
only harm, with no benefit. It is for this reason that rigorous testing is needed to prove that a screening test has efficacy (15). It is clear that there are palpable cancers that are not visible by mammography but are visible by ultrasound (16). What is not clear is how many cancers that are not palpable and not visible by mammography are detectable by ultrasound.
Although I have little doubt that there are cancers that are detectable only by ultrasound, this remains to be proven. If the ACRIN trial is performed properly it will provide important information on the sensitivity and specificity of ultrasound in a triple-blind evaluation of clinical breast examination, mammography, and ultrasound, with each investigator initially blinded to the findings of the others and then with knowledge of all the available information (this is the only way to avoid the significant potential for bias). The technique of scanning needs to be defined and reproducible. If ultrasound screening is to be feasible, it will likely require dedicated screening devices. It is not likely to be cost-effective to use hand-held systems for screening. The criteria for intervention must be carefully defined, because there are many ultrasound findings in any normal breast that could be mistaken for cancer and thus the false-positive rate could be extremely high.
Until the true ability of ultrasound to detect occult cancer is established, it should not be used outside of scientific trials for screening. Once it is established that ultrasound does have the ability to detect occult cancers, then it will need to be determined whether finding small cancers is sufficient to justify screening using ultrasound, or whether mortality reduction must first be established. Given the improvements in the technology that have taken place over the past years, ultrasound should be re-evaluated as a screening technique, but until its efficacy has been demonstrated, ultrasound should not be considered as a screening test.
Reliability of Using Ultrasound to Differentiate Benign From Malignant Masses
It has been said that “if your tool is a hammer, then everything is a nail.” There are enthusiasts in every field, and ultrasound is no exception. Many radiologists are highly proficient in the use of ultrasound in the evaluation of the breast. Numerous papers have been written about the various ultrasound criteria whose presence increases the likelihood that a lesion is benign or that suggest it is malignant. Most are not recent observations. Although Stavros is often credited with establishing criteria that might be used to differentiate benign from malignant lesions, most of the characteristics he used were developed decades ago. There is little doubt that oval or slightly lobulated, sharply marginated, well-circumscribed masses with homogeneous echotexture and enhanced through-transmission of sound that are longer than they are wide (the long access parallel to the skin) have a very high probability of being benign. The problem lies in the fact that cancer occasionally has all of these characteristics.
What has not been determined is how reliable a noninvasive test must be to avoid the need for tissue sampling (needle or excisional biopsy). As a consequence of the costs associated with breast biopsy (i.e., economic, psychological, and physical), there has been great emphasis placed on using imaging to avoid a biopsy. This is perfectly reasonable as long as the woman is informed of the options and has the opportunity to decide how much certainty she requires. Because the stakes may be as high as life and death, it should not be sufficient that a test such as ultrasound be able to predict the nature of a lesion, say, 70% of the time; this leaves too much room for error. Fortunately, a breast biopsy, even surgical excision, has an extremely low morbidity and virtually no mortality. The only biopsy that is safer is a skin biopsy. The cost of a breast biopsy can be reduced. If it is performed skillfully, there should be little trauma, and there need be little or no residual evidence of the needle biopsy, or even surgical biopsy. If ultrasound, or any other noninvasive test, is promoted as a method to differentiate benign from malignant lesions, the false-negative rate becomes critical, and women should be properly informed of the probabilities that the test result will be incorrect; they should also be provided with their options.
The real question lies in how accurately ultrasound can differentiate the borderline lesions, not the cancers that are obvious clinically or by mammography. There is no reason to use ultrasound to try to determine whether a spiculated lesion by mammography is benign or malignant. Nevertheless, the studies that have been used to suggest that ultrasound is good for separating benign lesions from malignant have included obvious cancers. Including these lesions only dilutes the data and makes ultrasound appear more accurate than it actually is. This is compounded by the fact that, once again, the ultrasound analyst has not been blinded to the mammographic findings and those from clinical breast examination. Knowing that a lesion is a spiculated mass by mammography, it would be unlikely for an investigator to suggest that it was a benign lesion by ultrasound.
How Accurate Is Accurate?
The uses of imaging in breast evaluation are unique. The only proven efficacy is from using mammography to screen asymptomatic women to reduce the mortality from breast cancer through earlier detection. Using ultrasound to differentiate cysts from solid masses is another efficacious use for sonography. Given the statistical probabilities and the fact that breast cancer is fairly rare relative to the number of women at risk, the vast majority of abnormalities found by mammography or clinical breast examination prove to be benign. Investigators should constantly be reminded that in a population of women being screened repetitively, there will only be two to four new cancers each year. When the accuracy of a test is considered, it should be remembered
that if 1,000 screening mammograms were reported as negative, without the radiologist even reviewing them, the accuracy would be 99.96% to 99.98%. This is a greater accuracy than virtually any other medical test. The problem, of course, is that this approach would have failed to detect the two to four cancers among the 1,000 mammograms. Although the test was highly “accurate,” it would actually have been totally without value and might have even had a negative impact if the reports were used to falsely reassure the women being screened.
that if 1,000 screening mammograms were reported as negative, without the radiologist even reviewing them, the accuracy would be 99.96% to 99.98%. This is a greater accuracy than virtually any other medical test. The problem, of course, is that this approach would have failed to detect the two to four cancers among the 1,000 mammograms. Although the test was highly “accurate,” it would actually have been totally without value and might have even had a negative impact if the reports were used to falsely reassure the women being screened.
What is usually overlooked when a new test is promulgated is the critical question, “How does use of the new test actually alter the care of the patient, and is this alteration beneficial for the patient?” Many papers and books describe how features seen on ultrasound can be used to predict whether a lesion is likely to be benign or malignant, but these are irrelevant if the lesion must still be biopsied or removed to provide certainty. If a test cannot safely alter the care of an individual, then its efficacy must be questioned.
In a highly quoted and referenced article, Stavros et al (17) claimed that ultrasound can accurately classify “some” solid lesions as being benign such that biopsy can be avoided. They presented criteria that they claimed permitted them to accurately differentiate benign lesions from malignant lesions (Table 17-1). Unfortunately, this publication was an unstructured report of a conglomerate of retrospective findings. The selection criteria for women who were evaluated were poorly specified and produced an aggregate of lesions that were inappropriately analyzed as a whole. As noted above, they included masses that were spiculated by mammography and for which ultrasound was not indicated diagnostically. They included lesions that were also typically benign, and there was no blinding as to the clinical and mammographic features of the lesions. Without the proper study design, the results are impossible to interpret and the conclusions are unsupported; however, many who use sonography rely on this classification.
TABLE 17-1 CHARACTERISTICS BY ULTRASOUND | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
The Stavros et al report was essentially a compilation of anecdotal observations. The authors speculated on the origin of ultrasound findings as if they were fact when in reality they have never been substantiated. For example, they stated that an echogenic border was indicative of infiltration. This is mere speculation. They suggested that “punctate calcifications that are sonographically visible within solid nodules are more likely to be associated with malignant than benign lesions.” This relationship has never been substantiated. They stated as fact that “markedly hyperechoic tissue that is well circumscribed and of uniform echogenicity represents fibrous tissue.” This has never been documented, and hyperechoic cancers, although rare, have been seen (personal communication from Alan Semine, MD).
The report also mixed palpable and nonpalpable lesions indiscriminately, which reflects the failure to appreciate the efficacious use of an imaging technique.
The majority of the criteria used by Stavros to differentiate benign lesions from malignant were nothing new. Most had been described over many years by other authors (18,19,20) and had been found to be insufficiently accurate to be used reliably. The authors added one new criterion: they described a thin echogenic rim that they felt was associated with benign masses (Fig. 17-6). The authors stated, “Sonography helped to correctly classify 100 nodules as malignant in comparison to 38 classified as malignant with mammography.” What they overlooked was the fact that comparing sonography to mammography as a diagnostic test is a meaningless endeavor. It is well established that
mammography is not a very accurate “diagnostic” technique; it is a screening test. The attempt to differentiate borderline lesions into benign versus malignant by imaging is meaningless, since all of these lesions require a tissue diagnosis, regardless of their ultrasound classification. Even though the analyst might suggest that these would prove to be malignant, there was no proof that a biopsy could be avoided. Once it is clear that a tissue diagnosis is needed, there is no value to additional imaging unless the additional study can safely eliminate some of that intervention. The real reason for using ultrasound to evaluate a solid mass is to determine whether it can be seen by ultrasound to decide if it can be biopsied under ultrasound guidance.
mammography is not a very accurate “diagnostic” technique; it is a screening test. The attempt to differentiate borderline lesions into benign versus malignant by imaging is meaningless, since all of these lesions require a tissue diagnosis, regardless of their ultrasound classification. Even though the analyst might suggest that these would prove to be malignant, there was no proof that a biopsy could be avoided. Once it is clear that a tissue diagnosis is needed, there is no value to additional imaging unless the additional study can safely eliminate some of that intervention. The real reason for using ultrasound to evaluate a solid mass is to determine whether it can be seen by ultrasound to decide if it can be biopsied under ultrasound guidance.
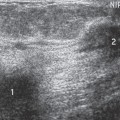
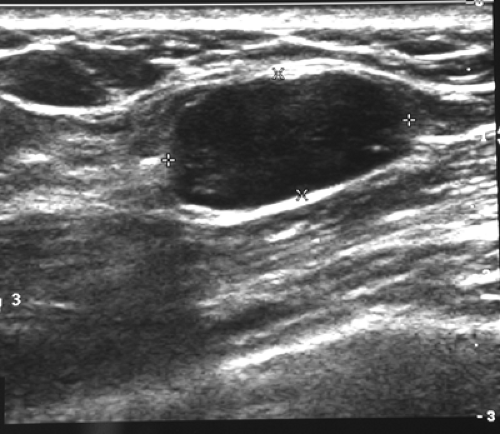 Figure 17-6 Some believe that a thin echogenic rim is the hallmark of a benign lesion such as this fibroadenoma. |
Another major problem with the Stavros paper is the fact that the age distribution of the patients with the various lesions was not provided. If a high proportion of the women with benign lesions were under age 30, then this would shift a large number of lesions to benign categories based on the age of the women alone without even looking at the images. If the radiologist who interpreted the images knew that the patient was under 30, then the image interpretation would likely be biased by this knowledge (cancer is extremely rare among women under age 30) and the results would be influenced. Including a large number of these patients would make the overall statistics seem better then they actually are by diluting the percentage of misses.
The real question is not whether ultrasound is helpful in evaluating spiculated lesions (virtually always malignant) or masses among women under age 30 (you can “bet the farm” that these latter cases are fibroadenomas, and imaging these women adds little to their care). The real question is whether ultrasound has any value in differentiating the borderline lesions (sharply marginated round, oval, or smoothly lobulated masses) or the lesions with ill-defined/obscured margins that raise concern.
In addition to having not been validated scientifically, the Stavros criteria do not add much to the sonographic evaluation of lesions. The authors required that to be classified as a benign lesion, the mass could not have any of the indeterminate or malignant features, despite the fact that these could all be seen with lesions that proved to be benign. The “echogenic capsule” can not only be seen with malignant lesions, but it is very uncommon even among benign lesions, especially if the fibroadenomas found among women under age 30 are removed from the analysis. In a study of 403 solid lesions that were biopsy-proven, 262 benign masses were included. Among these, only 66 (25%) had a thin echogenic pseudocapsule, and 3 of these (5%) were malignant lesions (21). Other criteria that the authors evaluated also showed the overlap between benign and malignant lesions using ultrasound criteria. There were 193 lesions with well-circumscribed margins. Among these, 20 (10%) were malignant. Among the 107 lesions that were “taller than wide,” 76 (71%) were malignant, but 31 (29%) were benign. Among the 91 lesions that had enhanced through transmission, 58 (64%) were benign, but 33 (36%) were malignant. This paper concluded that many of the descriptors used to try to differentiate benign from malignant lesions are, in total, “statistically significantly” different for differentiating all benign lesions from all malignant lesions. What the authors failed to explain is that the percentage of lesions with a given criterion might be significantly different when comparing all benign lesions with all malignant lesions, but this ignores the critical question, “Can the criterion be used to accurately determine whether a specific individual’s lesion is benign or malignant?” Since all criteria overlap, it is somewhat risky to rely on them to differentiate specific individual lesions.
Because we all would like to find imaging tests that differentiate benign from malignant lesions, and because many ultrasound enthusiasts do not appear to understand or wish to participate in scientific validation studies, there is the perception in the breast imaging community that ultrasound can be used to differentiate solid breast masses. It is important to realize that there is no scientifically clear support for this perception. The reader is cautioned that this capability has not been shown in any scientifically valid study.
Diagnostic Ultrasound
With the exception of a simple cyst, ultrasound is not diagnostic because there is a significant overlap in the characteristics of benign solid tissues and malignant lesions. Given the safety and ease with which a cytologic or histologic diagnosis can be achieved in the breast and the greater certainty afforded by having tissue confirmation, the value of tests that rely on probabilities to avoid more definitive diagnosis is questionable. Since most breast lesions are benign, one could decide without even looking at the lesion that is was benign, and the decision would have a high degree of accuracy. It is important to remember this when claims are made for a diagnostic breast evaluation test. Ultimately, it is the patient and her physician who must decide how much certainty is required.
Doppler Ultrasound
Numerous attempts have been made using Doppler ultrasound to improve the accuracy of breast evaluation. Because many breast cancers appear to have a substantial neovasculature (at the microscopic level), and most benign lesions do not have increased blood flow, the rationale for Doppler would appear to be justified. The clinical results, however, have not been particularly convincing. Many cancers can be shown to have increased blood flow, particularly at their periphery. High-velocity flow seems to be found almost only in breast cancers (22). Although preliminary reports suggested a high sensitivity and specificity for
Doppler (23), subsequent studies were not as successful, suggesting poor discrimination between normal and cancerous tissues (24). The most optimistic report, by Cosgrove et al (25), concluded that a “color Doppler signal in a lesion otherwise thought to be benign should prompt a biopsy, while the absence of signals in an indeterminate lesion is reassuring.” The clinical application of this is not particularly useful. It might provide additional reassurance for women with lesions that are thought to be benign, but depending on the lesions placed in this category, the use of Doppler would only add to the cost of care because these lesions would otherwise be left alone. Indeterminate lesions would still require cytologic or histologic confirmation. Another problem with the Cosgrove et al report was that the lesions were palpable, and evaluation of smaller lesions has not proved to be as successful.
Doppler (23), subsequent studies were not as successful, suggesting poor discrimination between normal and cancerous tissues (24). The most optimistic report, by Cosgrove et al (25), concluded that a “color Doppler signal in a lesion otherwise thought to be benign should prompt a biopsy, while the absence of signals in an indeterminate lesion is reassuring.” The clinical application of this is not particularly useful. It might provide additional reassurance for women with lesions that are thought to be benign, but depending on the lesions placed in this category, the use of Doppler would only add to the cost of care because these lesions would otherwise be left alone. Indeterminate lesions would still require cytologic or histologic confirmation. Another problem with the Cosgrove et al report was that the lesions were palpable, and evaluation of smaller lesions has not proved to be as successful.
Because benign lesions may exhibit increased flow and, more important, a significant number of cancers do not exhibit evidence of abnormal flow (26)—particularly lesions smaller than 1 cm (27)—Doppler is not yet reliable for distinguishing benign lesions from malignant lesions. There is the perplexing overlap of benign and malignant characteristics on Doppler analysis that makes it difficult to avoid obtaining cytologic or histologic material (a biopsy).
Some find Doppler useful when evaluating lesions that are borderline in their morphologic characteristics. Even modern ultrasound units put echoes into lesions that likely represent cysts. If vasculature can be demonstrated within such a mass, then it is clearly not a cyst. Unfortunately, the absence of vasculature within such a lesion does not mean that it cannot be solid. For such borderline lesions, we prefer ultrasound-guided aspiration or core needle biopsy. In general, Doppler is not a clinically useful test for evaluating breast lesions.
Intravenous Enhancement of Breast Lesions for Ultrasound Evaluation
There is some hope that, just as with computed tomography, digital angiography, and MRI, the neovascularity that develops with many cancers can be demonstrated by using intravenous (IV) contrast agents that enhance the visibility of vessels on ultrasound. Several papers have been published suggesting that contrast material can enhance the visiualization of blood vessels under ultrasound observation. The problem lies in whether the use of these agents actually aids in managing patients with breast lesions. As with many preliminary reports, one using the IV infusion of microbubbles was encouraging (28), but this type of evaluation is still in development. At present contrast-enhanced breast ultrasound remains experimental, with no proven clinical efficacy.
Ultrasound and Women with Radiographically Dense Breast Tissues
As noted in the section “Ultrasound as a Screening Technique,” there have been a number of reports that ultrasound might be useful as a second-level screen for women who have radiographically dense tissues that could obscure a lesion on mammography. It is fairly well established that some palpable lesions that are obscured by surrounding tissues are not visible by mammography. These are frequently visible by ultrasound. However, until blinded evaluation of screening (detection of occult cancer) ultrasound demonstrates the true sensitivity of the test, it is not clear how valuable ultrasound could be as a screening study. Just as with mammographic screening, it is likely that a randomized, controlled trial will be needed to determine whether screening with ultrasound saves lives and if the high false-positive rate is justified. Until the efficacy of ultrasound to screen any population of women, including those with radiographically dense breasts, has been demonstrated in properly performed studies, it should not be used as a screening test outside of clinical trials. My belief is that ultrasound will be shown to be able to detect some small cancers that are occult to both clinical examination and mammography and that it will be shown to be efficacious as a second-level screening test, but “beliefs” can be completely incorrect and are no substitute for properly performed scientific studies.
The Appropriate Use of Breast Ultrasound
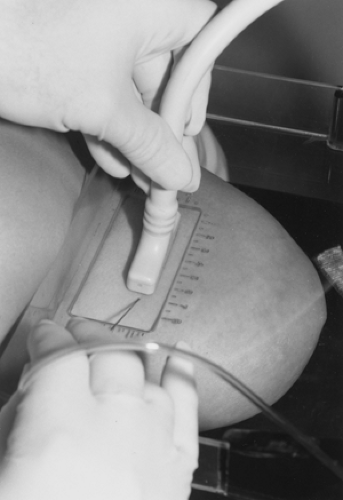
Ultrasound should be used as a technique to help resolve specific management questions. If a lesion is not palpable but is detected by mammography, ultrasound can be used to analyze its composition. In particular, the ultrasound demonstration that a mammographically detected lesion is a simple cyst obviates the need for surgery or even further intervention (Fig. 17-7). If a lesion meets the strict ultrasound criteria for a cyst, the study is “diagnostic.” If there is any question about the concordance of the lesion seen by ultrasound with the density on mammography, a guided aspiration followed by repeat mammography can resolve the question. Introducing a small volume of air (less than the volume of fluid removed, to avoid pain) can confirm that the lesion seen using mammography was the lesion aspirated using ultrasound. A needle can also be placed through the lesion under ultrasound and its relationship to the mammographic finding confirmed by mammography. We have found it helpful, on occasion, to evaluate the lesion with ultrasound while the breast is held in the mammography unit. The lesion is positioned in the window of the fenestrated compression paddle and ultrasound examination can be performed thorough the window in the paddle
(Fig. 17-8). In this way direct correlation can be made using the mammography coordinates from the window grid and the position of the ultrasound transducer in the window.
(Fig. 17-8). In this way direct correlation can be made using the mammography coordinates from the window grid and the position of the ultrasound transducer in the window.
Ultrasound can occasionally help in the triangulation of lesions found in only one mammographic view. Ultrasound is very useful for guiding the aspiration of cysts (Fig. 17-9) and for obtaining cytologic material using fine-needle aspiration (29,30). With the development of ultrasound systems that permit the operator to view the biopsy needle and lesion together in real time, ultrasound has become the imaging method of choice for guiding core needle biopsies of masses in the breast (31,32,33). As with any imaging-guided needle biopsy, there is the potential for sampling error, and the results of an ultrasound-guided biopsy should be compared to the imaging to ensure that they are concordant with what was suggested by the imaging. If they are discordant, a repeat biopsy or excision of the lesion is recommended.
Ultrasound can also be used to position needles and wire guides for preoperative localization before surgical excision of sonographically visible lesions (34,35,36). Needle localization using ultrasound can be accomplished in a matter of minutes and is more comfortable for the patient then having her breast compressed for a mammographically guided localization, and we will use sonography for these needle localizations whenever possible.
Ultrasound and Implants
There are no data supporting the use of ultrasound to screen women with implants to detect breast cancer. Ultrasound
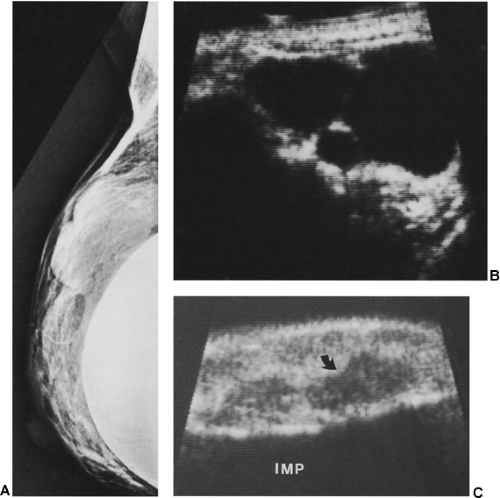
may be helpful in evaluating women with implants if they have palpable lesions discovered by physical examination or for lesions found by mammography to determine whether such lesions are cystic or solid (Fig. 17-10). Ultra-sound is used to avoid inserting a needle, which might damage the implant. Some have found ultrasound helpful in trying to determine whether an implant is ruptured (37).
may be helpful in evaluating women with implants if they have palpable lesions discovered by physical examination or for lesions found by mammography to determine whether such lesions are cystic or solid (Fig. 17-10). Ultra-sound is used to avoid inserting a needle, which might damage the implant. Some have found ultrasound helpful in trying to determine whether an implant is ruptured (37).
When silicone implants rupture, the silicone can be extruded into the surrounding tissues. Many implants become surrounded by a fibrous capsule. When these rupture, the extruded silicone may still be contained within the fibrous capsule; this is called an intracapsular rupture. If the implant ruptures and the silicone passes through an incomplete or torn fibrous capsule, the result is called an extracapsular rupture.
If an implant ruptures and silicone is extruded into the surrounding tissues, its appearance can be quite typical on ultrasound: it can produce a “snowstorm” of echogenicity with posterior loss of information that is fairly specific in its appearance (Fig. 17-11) (38). Rosculet et al believe that this echogenic noise occurs when the silicone globules are so small that they are the size of a wavelength of the sound (39). Due to differences between the speed of sound in the silicone and breast tissue (sound travels slower in silicone), they hypothesize that focal areas of the beam are advanced and others are delayed as the beam passes through the tissues interspersed with silicone, and the beam becomes out of phase with the surrounding tissue. With loss of coherence, the result is a snowstorm of noise. Large globules of silicone, on the other hand, may produce hypoechoic masses. The silicone and the fibrous reaction to it from the tissues outside of the implant can cause areas of shadowing as well.
The term “rupture” sounds fairly dramatic, but most ruptures are actually tears in the silicone rubber envelope that contains the viscous silicone gel of the implant, allowing the silicone to ooze out of the implant. What frequently happens is that the envelope tears and rather than the silicone oozing out, the rubber envelope literally collapses into the gel, which still remains held in place within the previously formed fibrous capsule. This intracapsular rupture may appear normal on mammography because the contour of the silicone gel remains smooth because it is contained by the fibrous capsule.
One of the more useful signs of an intracapsular rupture has been described on both MRI and ultrasound. As the envelope collapses into the gel, it folds on itself, and these folds can appear as a “stepladder” on ultrasound (Fig. 17-12). DeBruhl et al (40) found that this was the most reliable sign of rupture in a series of 74 women with implants evaluated by ultrasound. The stepladder appearance was noted in 14 of the 20 known ruptured implants.
We find it impractical to use ultrasound to survey the entire surface of the implant to determine whether it is ruptured. MRI is superior to ultrasound, and we prefer to use MRI to evaluate implants for possible rupture.
The importance of detecting implant ruptures has become less urgent. An impartial review by leading experts failed to show any evidence that implant rupture was associated with any systemic illnesses, as some had alleged (41).
Ultrasound and Asymmetric Breast Tissue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree