Abstract
Bullous emphysema, often associated with COPD, can lead to severe complications like massive hemoptysis. The Bronchial artery embolization (BAE) has become a well-established and effective procedure for the management of hemoptysis, which is the expectoration of blood from the lower respiratory tract. First introduced in the 1970s, BAE has evolved significantly due to advancements in interventional radiology techniques and embolic materials. The success rate of BAE in controlling acute hemoptysis ranges from 70% to 90% in the literature. However, recurrence rates remain a challenge, with studies reporting recurrence in up to 20%-30% of cases within the first year, often due to incomplete embolization or disease progression. Repeat embolization is frequently required in these patients, highlighting the importance of close follow-up and management of the underlying disease.
This case report describes a 55-year-old patient with a history of pulmonary tuberculosis, chronic smoking, and advanced COPD who presented with significant hemoptysis due to a hemorrhagic emphysematous bulla. Due to the patient’s fragile condition, surgical intervention was deemed too risky, and embolization was chosen as a less invasive alternative. The procedure successfully controlled the bleeding without complications. This case highlights the importance of bronchial artery embolization (BAE) as a life-saving intervention in cases of massive hemoptysis, particularly in patients unfit for surgery. While BAE provides an effective solution for acute bleeding, long-term management of COPD and close follow-up are essential to prevent recurrence. A multidisciplinary approach is crucial for optimal patient outcomes.
Introduction
Bullous emphysema, a complication of COPD, typically presents with pneumothorax. However, in rare cases, fluid accumulation within the bullae can lead to life-threatening complications, such as massive hemoptysis. We report a rare case of a 55-year-old patient with a history of pulmonary tuberculosis, chronic smoking, and advanced COPD, who presented with significant hemoptysis due to a large cystic lesion inside an emphysematous bulla. Despite extensive investigations ruling out infectious and embolic causes, the patient’s fragile condition made surgical intervention impossible. This case highlights the importance of a comprehensive approach, which includes a thorough patient history, careful imaging analysis, and collaboration among a multidisciplinary team to effectively manage complex COPD-related complications.
Case report
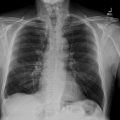
A 55-year-old patient with a history of pulmonary tuberculosis, chronic smoking, and COPD on dual bronchodilation therapy LABA (Long-Acting Beta-Agonist) and LAMA (Long-Acting Muscarinic Antagonist) presented with chronic hypercapnic respiratory failure managed with long-term oxygen therapy and home noninvasive ventilation. The patient also had a history of cor pulmonale, which was managed with diuretics to alleviate fluid overload and reduce right ventricular strain. The patient was admitted to the hospital due to worsening dyspnea and significant hemoptysis, without a history of trauma ( Fig. 1 ). On physical examination, his oxygen saturation was 77% on room air, improving to 92% with 2L of oxygen via nasal cannula. He exhibited tachypnea at 25 breaths per minute and tachycardia at 105 beats per minute. Auscultation of the lungs revealed diminished breath sounds bilaterally, with no adventitious sounds such as rales, wheezing, or rhonchi detected. There was no evidence of stridor, and vocal resonance was unremarkable. The findings suggest reduced air entry, potentially indicative of underlying alveolar or pleural pathology

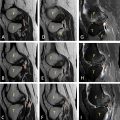
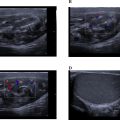
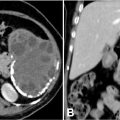
Laboratory results showed leukocytosis of 9,910 cells/μL, platelets at 233,000 cells/μL, a prothrombin time (PT) ratio of 100%, D-dimer levels of 360 ng/mL, and a C-reactive protein (CRP) of 16.5 mg/L. thoracic CT scan without and with contrast had demonstrated a emphysematous bulla in the middle lobe with hyperdense hemorrhagic content, noting the presence of surrounding ground-glass opacities corresponding to alveolar hemorrhage ( Fig. 2 ). Bedside ultrasound revealed a heterogeneous, unvascularized cystic lesion on Doppler imaging with peripheral air-filled bullae.

A subsequent CT pulmonary angiogram (CT-PA) during admission revealed a large cystic structure measuring 73 × 58 × 107 mm, located in the lateral segment of the middle lobe, with an air-fluid level suggestive of a hemorrhagic bulla. No bleeding source or arteriovenous malformation was identified ( Fig. 2 ).
Initial management included the intravenous administration of tranexamic acid at a dose of 1 gram every 8 hours and etamsylate at a dose of 500 mg every 6 hours to control hemoptysis resulting in a significant reduction in hemoptysis within five days of admission. The decision was made to pursue a less invasive approach with endovascular treatment, opting for systemic embolization.
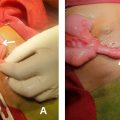
The procedure was initiated via femoral vascular access using a 5F introducer, allowing the advancement of a Simmons-type navigation catheter. Selective catheterization of the right broncho-intercostal trunk revealed, upon contrast injection, a localized vascular blush in the middle lobe. Superselective catheterization of the right bronchial artery was then performed using a Progreat 2.9 Fr microcatheter, followed by embolization with 1 ml of 800 μm microparticles ( Fig. 3 ) until a significant reduction in arterial flow was achieved. Postembolization angiographic control confirmed the complete resolution of the vascular blush ( Fig. 4 ).