Cardiac Radiology
Pal Suranyi
Yeong Shyan Lee
Joseph U. Schoepf
Case 10.1
HISTORY: A 57-year-old man with known history of triple vessel disease
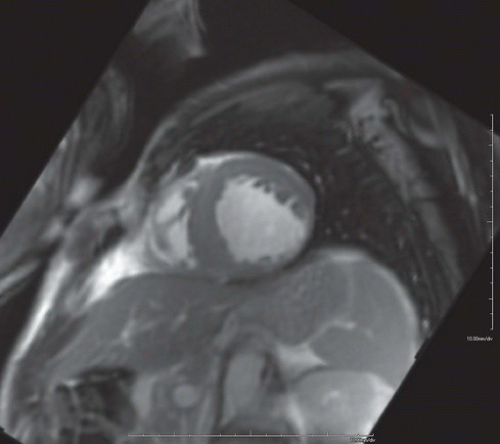
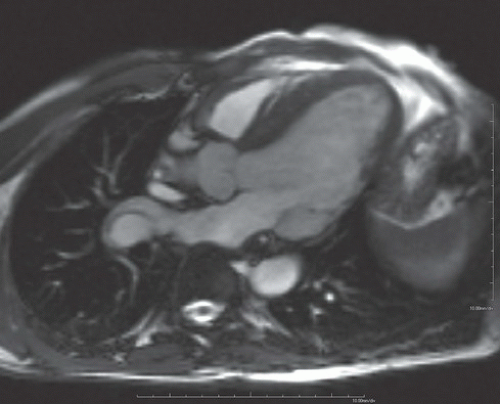
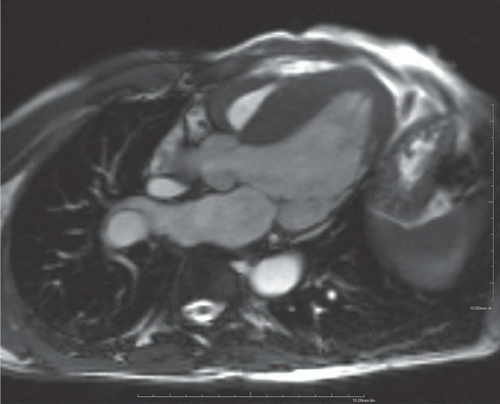
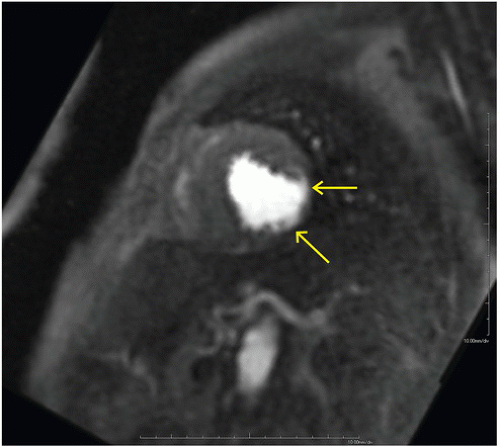
FINDINGS: Diastolic (Fig. 10.1.1) and systolic (Fig. 10.1.2) short axis steady-state free precession (SSFP) MRI images show focal akinesis and wall thinning of the lateral-inferolateral wall of left ventricle. These findings are confirmed on three-chamber long axis (left atrium, left ventricle, and aorta) diastolic and systolic views (Figs. 10.1.3 and 10.1.4). The short axis first-pass perfusion image (Fig. 10.1.5) shows hypoenhancement (arrows) in these segments of the left ventricular wall, compatible with hypoperfusion. Phase-sensitive inversion recovery (PSIR) image shows corresponding delayed transmural hyperenhancement (Fig. 10.1.6, arrows) 15 minutes following gadolinium-based contrast agent injection.
DIAGNOSIS: Chronic myocardial infarct at the left circumflex artery territory with scarring and associated wall thinning and focal segmental wall akinesis
DISCUSSION: Both myocardial ischemia and myocardial infarction appear hypointense on first-pass perfusion images. The viability of the region at question can be evaluated by delayed enhancement imaging (DE-MRI), where the signal from viable myocardium is minimized (nulled) by selecting an appropriate delay following an inversion recovery pulse. Hyperenhancement occurs in both acute infarction and chronic scarring. Hyperenhancement
can range from subendocardial (Fig. 10.1.7), suggesting better prognosis owing to residual epicardial viable tissue, to transmural, indicating the absence of residual (salvageable) viable myocardium. The extent of transmurality has been shown to be a strong prognostic indicator for recovery of contractility following revascularization and medical therapy in patients with ischemic heart disease and left ventricular systolic dysfunction (1).
can range from subendocardial (Fig. 10.1.7), suggesting better prognosis owing to residual epicardial viable tissue, to transmural, indicating the absence of residual (salvageable) viable myocardium. The extent of transmurality has been shown to be a strong prognostic indicator for recovery of contractility following revascularization and medical therapy in patients with ischemic heart disease and left ventricular systolic dysfunction (1).
Localization of the infarct to specific coronary artery distribution is also made possible by demonstrating the wall motion abnormality in the cine MRI, although resting function by itself is not a reliable indicator of viability or the lack thereof.
Aunt Minnie’s Pearls
Both ischemic and infarcted myocardium may present as hypoenhanced regions on a perfusion scan with wall motion abnormality in the same region. The presence or absence of delayed enhancement decides viability.
The greater the transmurality of delayed enhancement, the worse the outcome following revascularization.
Case 10.2
HISTORY: Two patients, with chest pain and abnormal Q waves and ST segment elevation on the ECG, a few weeks posttrauma. Figures 10.2.1A, B are MRI images of the first patient, whereas Figures 10.2.2A, B are CT images of the second patient.
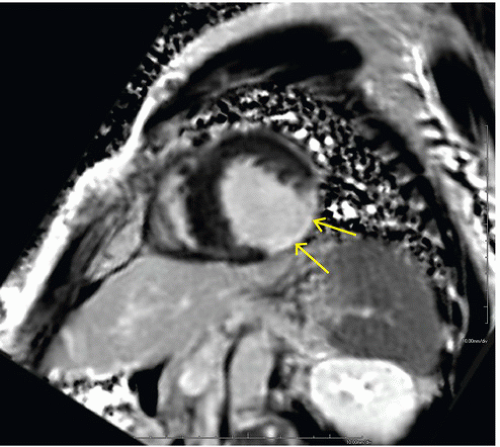
FINDINGS: Four-chamber SSFP cine MR images in diastolic and systolic phases (Figs. 10.2.1A, B) show an abnormal bulge (arrows) at the right ventricular anterior wall, which is accentuated in the systolic phase. The pericardium is well separated from this abnormal ventricular bulge. Right pleural effusion and left basilar lung collapse are also demonstrated in these two MR images.
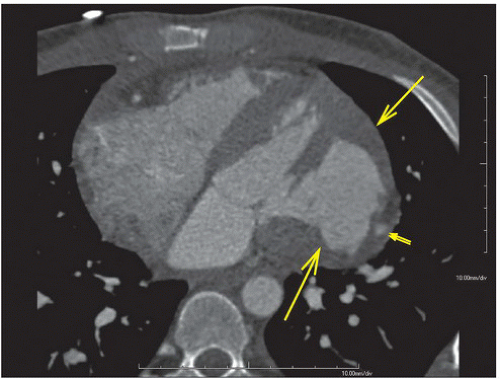
Axial and reformatted coronal CT scans of the second patient show a large abnormal bulge that is lined by a thin layer of myocardium in the free wall of the left ventricle (Figs. 10.2.2A, B, arrows). Also noted is focal dilatation of a coronary artery (double arrows) within the injured myocardium, suggesting the presence of traumatic coronary artery pseudoaneurysm.
DIAGNOSIS: Posttraumatic ventricular aneurysm
DISCUSSION: A true ventricular aneurysmal sac is lined by three layers of the heart wall, that is, the endocardium, the myocardium (and/or scar), and the epicardium, whereas a pseudoaneurysm sac comprises the epicardium and/or the pericardium containing blood from a ruptured ventricle. Both
types may present as rare complications of myocardial infarction and may contain mural thrombi. Ventricular pseudoaneurysms may also be caused by chest trauma, cardiac surgery, or endocarditis. It is traditionally believed that a false aneurysm has a higher risk of delayed rupture. A correct diagnosis and differentiation between the two conditions is therefore important as surgery is the definite treatment of choice for cardiac pseudoaneurysm.
types may present as rare complications of myocardial infarction and may contain mural thrombi. Ventricular pseudoaneurysms may also be caused by chest trauma, cardiac surgery, or endocarditis. It is traditionally believed that a false aneurysm has a higher risk of delayed rupture. A correct diagnosis and differentiation between the two conditions is therefore important as surgery is the definite treatment of choice for cardiac pseudoaneurysm.
A true ventricular aneurysm is commonly located in the anterolateral or apical wall and has a wide ostium connecting the sac to the ventricle, whereas a pseudoaneurysm is more commonly located in the posterolateral wall with a wide mouth. Injured, contused, and edematous myocardium lining a true aneurysm may show delayed enhancement, although pseudoaneurysms have also been shown to demonstrate marked delayed pericardial enhancement on MRI (2).
Aunt Minnie’s Pearls
True aneurysms are lined by endocardium, scarred myocardium, and epicardium and have wide ostia.
Pseudoaneurysms are lined by epicardium or pericardium and usually have narrow ostia.
Case 10.3
HISTORY: CT scan of a 26-year-old man following left internal mammary artery to left anterior descending artery (LAD) bypass grafting at age 11 owing to LAD-territory ischemia
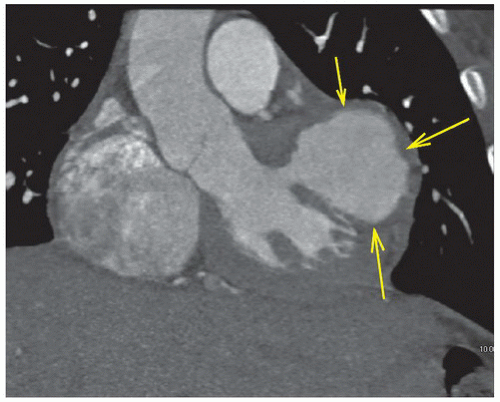
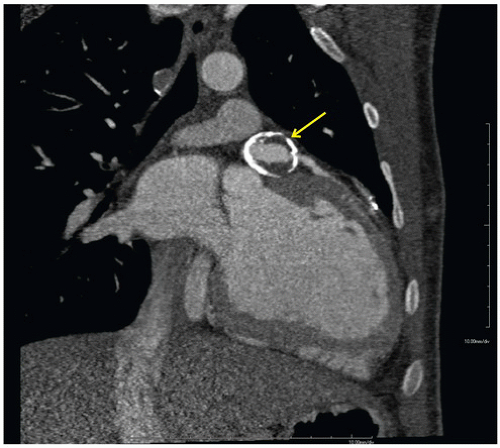
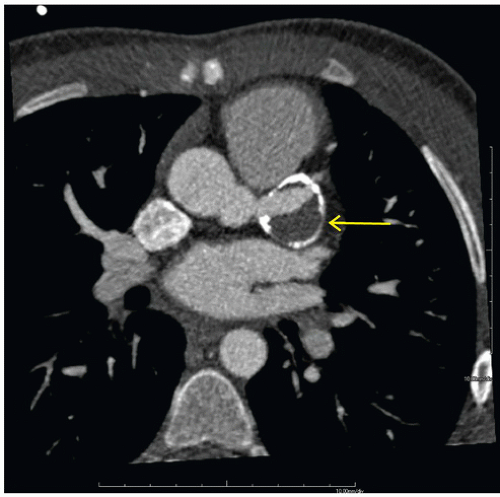
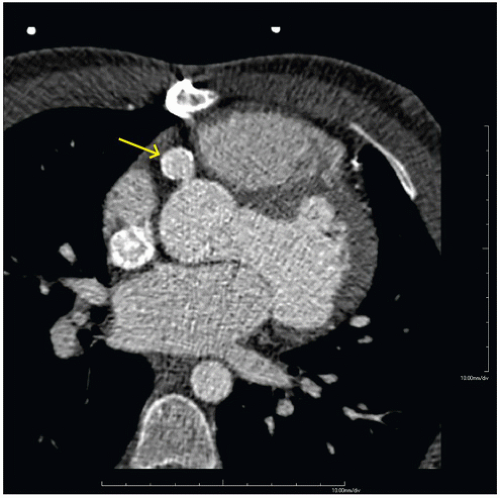
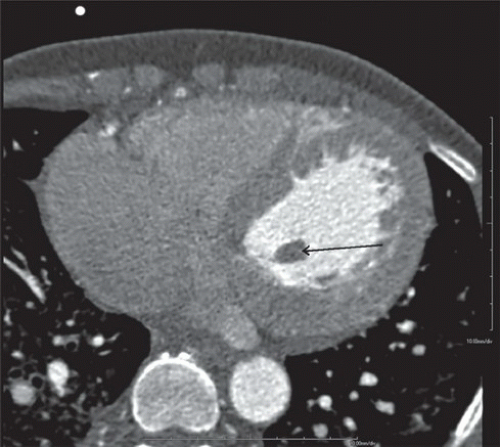
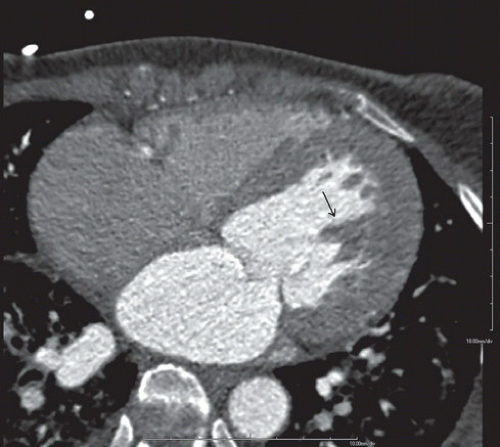
FINDINGS: Figures 10.3.1A, B show a large calcified aneurysm (arrows) with mural thrombus in the proximal left main coronary artery. Figure 10.3.2 shows another large calcified aneurysm in the proximal right coronary artery (arrow).
DIAGNOSIS: Kawasaki disease with coronary artery aneurysms
DISCUSSION: The major cause of coronary artery aneurysms in children and young adults is Kawasaki disease (mucocutaneous lymph node disease), an acute vasculitis of unknown etiology, affecting medium-sized arteries. It was first described by Dr. Tomisaku Kawasaki in 1967 (3). Most cases occur between 6 months and 8 years of age. These patients present with fever and swollen cervical lymph nodes and may have ischemic chest pain and ECG abnormalities.
Coronary aneurysms are believed to occur in 25% of children with Kawasaki disease (4). The coronary artery aneurysms generally occur within 3 to 6 months of the onset of the acute illness. Small (<5 mm) and medium-sized coronary aneurysms (5-8 mm) may regress, but giant aneurysms (>8 mm) remain unchanged or may even progress to thrombosis, stenosis, and myocardial infarction. Early treatment of Kawasaki disease with aspirin and high-dose intravenous gamma globulin is effective in reducing the formation of coronary artery aneurysms.
Aunt Minnie’s Pearls
Coronary artery aneurysms are believed to occur in 25% of Kawasaki disease.
Giant aneurysms have lower rate of regression, higher risks of stenosis, and strongest association with myocardial infarction.
Always keep Kawasaki disease in the back of your mind when you do imaging for symptoms of ischemic heart disease in a child.
Case 10.4
HISTORY: A 50-year-old asymptomatic adult with mid-systolic murmur that alters with position
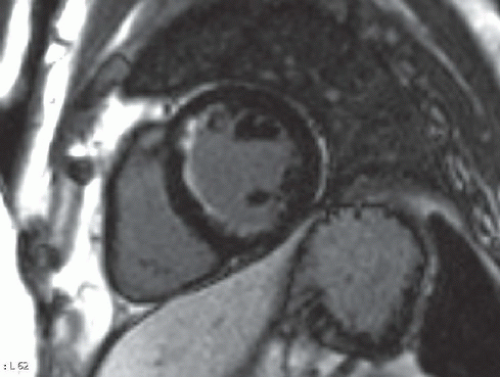
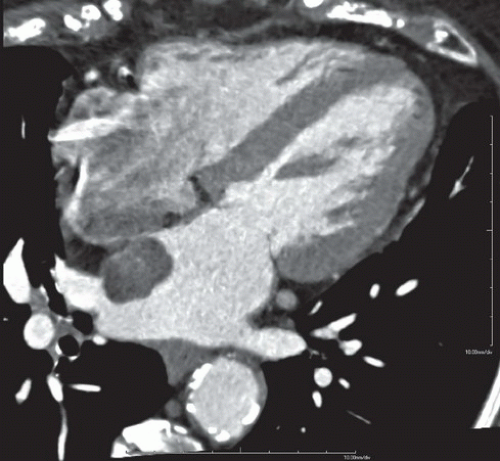
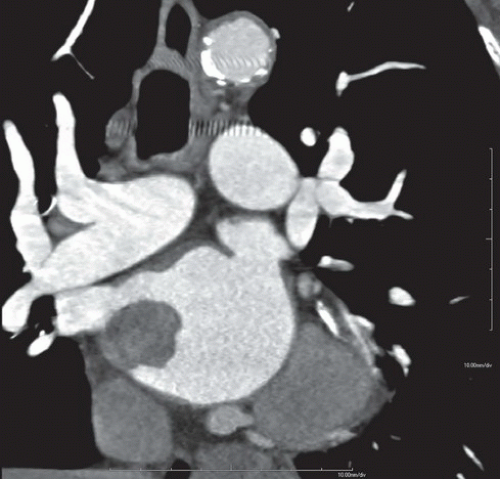
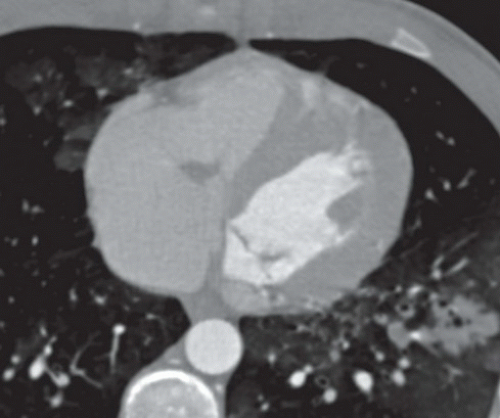
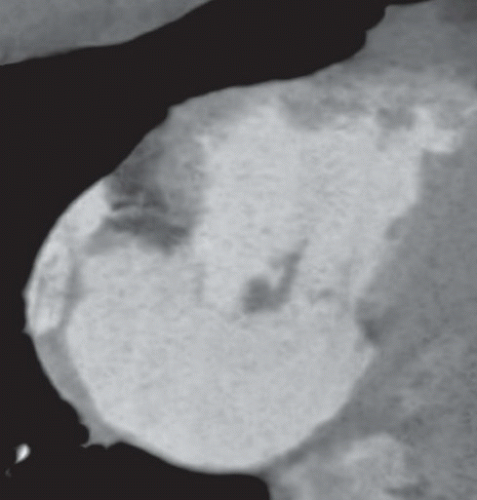
FINDINGS: Figures 10.4.1A, B show an ovoid hypodense mass with well-defined, slightly lobulated margin and mild contrast enhancement in the left atrium, adjacent to the interatrial septum.
DIAGNOSIS: Cardiac myxoma
DISCUSSION: Cardiac myxomas are the most frequent primary benign tumors of the heart. There is slight female predominance (5:4), and they usually occur between the age of 30 and 60 years. Patients may present with symptoms of mitral valve obstruction (cardiac failure or malaise), central embolism, and constitutional symptoms (fever, weight loss, or symptoms resembling connective tissue disorder owing to cytokine secretion) (5).
Most cardiac myxomas are sporadic but up to 7% of cases are familial. The so-called Carney complex is an autosomal dominant (AD) syndrome, characterized by multiple cardiac myxomas, spotty skin pigmentation, endocrine hyperactivity, and nonmyxomatous extracardiac tumors.
Cardiac myxomas are commonly located in the left atrium (75%-80%) and usually arise from the fossa ovalis region of interatrial septum. The tumors are often gelatinous in appearance owing to the abundance of myxoid matrix. They could either be broadly based or have a narrow stalk (pedunculated).
On CT scan, myxomas are usually well-defined, smooth or lobulated, intracavitary cardiac masses and typically contain calcifications. They are usually of lower density than the myocardium and show heterogeneous contrast enhancement. Sometimes cardiac myxomas may mimic thrombi.
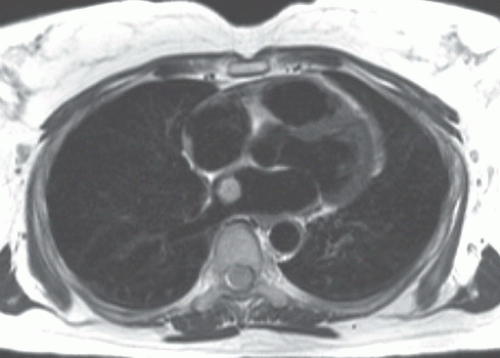
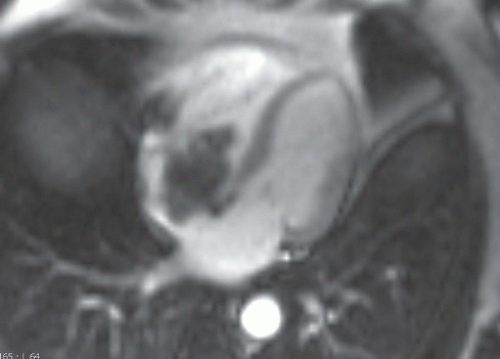
On MRI, myxomas are generally isointense with the myocardium in T1-weighted and hyperintense in T2-weighted images (Fig. 10.4.2). They might show areas of low-signal intensity on T2-weighted images owing to intralesional calcifications or hemosiderin.
Post-gadolinium, the lesion often show heterogeneous enhancement (Fig. 10.4.3). Most often they are in the left atrium where they often have round, smooth contours (Fig. 10.4.2). Less commonly myxoma may present as a right atrial mass, which is more likely to be lobulated (Fig. 10.4.3).
Aunt Minnie’s Pearls
Multiple myxomas + Spotty skin pigmentation + endocrine hyperactivity + nonmyxomatous extracardiac tumors = Carney complex (AD).
Myxoma may mimic thrombus. Lack of contrast enhancement makes thrombus more likely, especially given the low incidence of primary cardiac tumors.
Case 10.5
HISTORY: A 70-year-old female with chest pain, acute onset of hypotension and pulmonary edema
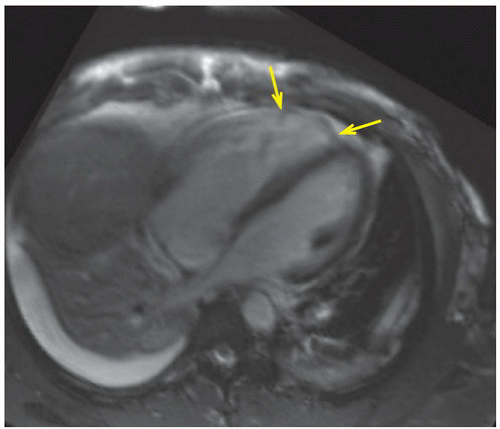
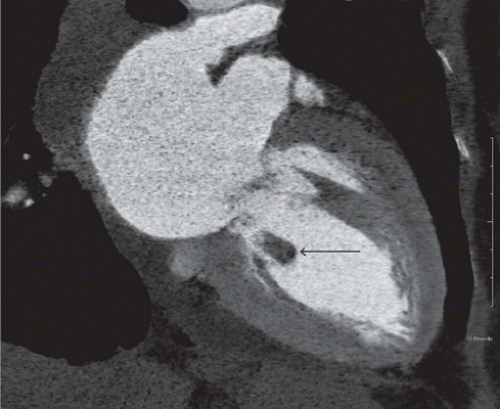
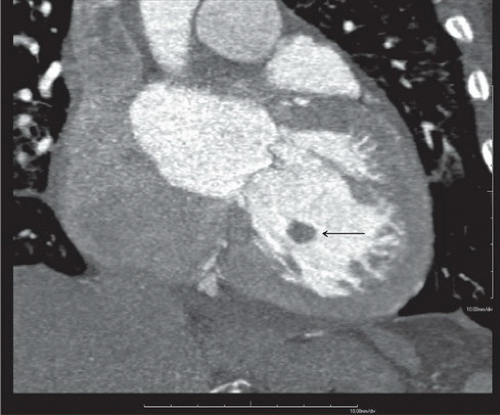
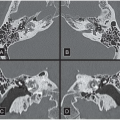
FINDINGS: Axial and reformatted coronal cardiac CT images (Figs. 10.5.1A, B, arrow) show a nodular mass in the left ventricle. It shows similar (although slightly lower) attenuation to the myocardium and is better demonstrated with a reconstructed oblique coronal MinIP (minimum intensity projection) image (Fig. 10.5.2, arrow). By careful examination of the cardiac anatomy, one can easily identify it as a disrupted posteromedial papillary muscle that is now contracted. The normal anterior lateral papillary muscle is demonstrated in the axial image (Fig. 10.5.3).
DIAGNOSIS: Ruptured papillary muscle
DISCUSSION: Acute rupture of the papillary muscle is uncommon. The causes of rupture of the papillary muscle include acute myocardial infarction (AMI) and cardiac contusion.
Acute papillary muscle rupture usually occurs 2 to 7 days following AMI, and it accounts for 5% of mortality of AMI (6). Acute papillary muscle rupture usually occurs with inferior wall myocardial infarction, and the posteromedial papillary muscle is generally involved owing to the posterior descending coronary artery being its single blood supplying vessel (7). The anterolateral papillary muscle has dual blood supply from both the left anterior descending and left circumflex coronary arteries. Patients usually present with acute, severe mitral regurgitation and might be hemodynamically unstable. In rare instances, papillary muscle rupture can happen in the right ventricle as well, causing severe acute tricuspid regurgitation (Figs. 10.5.4A, B) Prompt recognition of papillary muscle rupture is important as surgical repair could be lifesaving.
Aunt Minnie’s Pearls
Acute papillary muscle is uncommon but a potentially fatal complication of AMI.
High index of suspicion, early detection, and prompt surgical repair could be lifesaving.
MinIP helps visualize low-attenuation structures (chordae tendineae, papillary muscles, valves, dissection membranes).
Case 10.6
HISTORY: A 32-year-old man with chest pain and abnormal myocardial perfusion SPECT
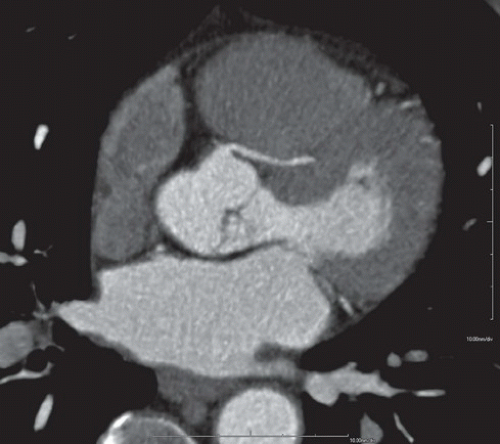 FIGURE 10.6.1
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|