Cardiovascular and Interventional Radiology
Stephen P. Loehr
The authors and editors acknowledge the contribution of the Chapter 3 author from the second edition: Adam Henn, MD.
Case 3.1
HISTORY: Two patients with sudden onset of pain radiating to the back. Figures 3.1.1 and 3.1.2 are of the first patient; Figure 3.1.3 is of the second patient.
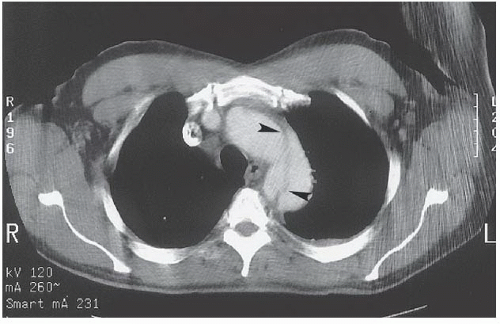
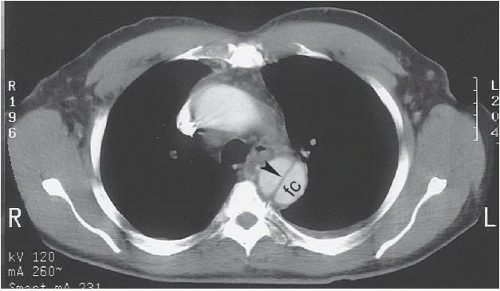
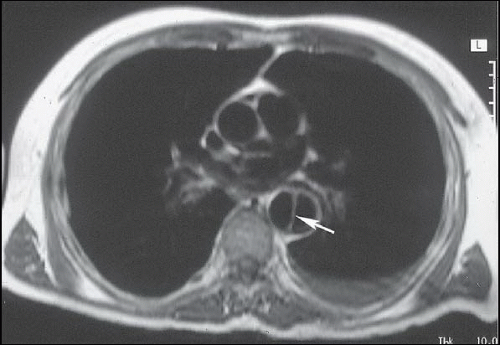
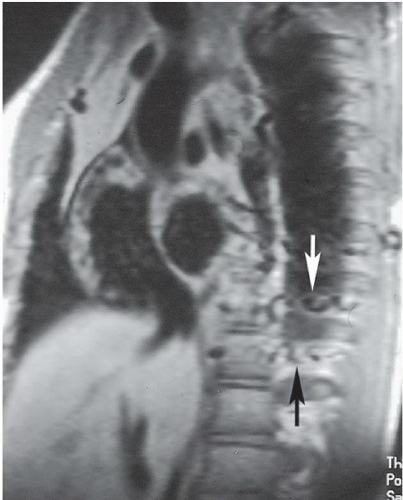
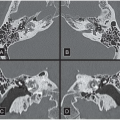
FINDINGS: Axial contrast-enhanced CT images through the chest reveal an intimal flap within the aortic lumen that originates near the left subclavian artery (Fig. 3.1.1, arrowheads) and extends inferiorly down the thoracic aorta (Fig. 3.1.2, arrowhead). The larger, false channel (fc) is located in the posterolateral descending aorta. The ascending aorta is normal. No active extravasation of contrast material from the aorta is identified. In a different patient with the same abnormality, an axial echocardiography (ECG)-gated, T1-weighted magnetic resonance imaging (MRI) also demonstrates an intimal flap (Fig. 3.1.3, arrow).
DIAGNOSIS: Aortic dissection (i.e., DeBakey type III or Stanford type B)
DISCUSSION: Dissection of the aorta occurs when a pathologic communication develops between the true aortic lumen and the aortic wall. The most common predisposing factor to the development of aortic dissection is hypertension (1). Other predisposing factors include Marfan and Ehlers-Danlos syndromes, pregnancy, trauma, aortic valve disease, and coronary artery bypass surgery (2). Dissections that involve the ascending aorta (i.e., DeBakey types I and II and Stanford type A) can lead to rapid
death because of rupture into the pericardium, acute valvular insufficiency, or coronary artery occlusion (3). These types of dissections are managed with emergent surgery (4). Dissections that involve only the descending aorta distal to the left subclavian artery (i.e., DeBakey type III or Stanford type B) are usually managed medically by control of the patient’s hypertension. Aortic dissections in selected patients have been successfully treated using endovascular methods (i.e., stent-grafts) (5,6).
death because of rupture into the pericardium, acute valvular insufficiency, or coronary artery occlusion (3). These types of dissections are managed with emergent surgery (4). Dissections that involve only the descending aorta distal to the left subclavian artery (i.e., DeBakey type III or Stanford type B) are usually managed medically by control of the patient’s hypertension. Aortic dissections in selected patients have been successfully treated using endovascular methods (i.e., stent-grafts) (5,6).
Aunt Minnie’s Pearls
Emergent surgery is required for Stanford type A dissections.
Always check for aortic rupture and branch vessel involvement.
If dissection involves the ascending aorta, look for pericardial hematoma.
Case 3.2
HISTORY: Cyanosis
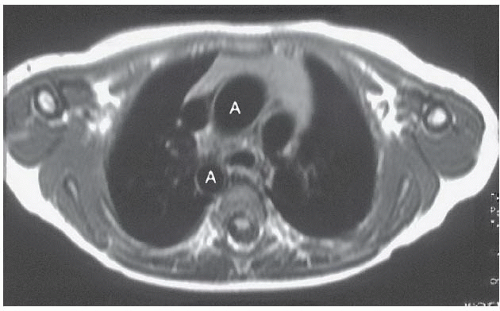
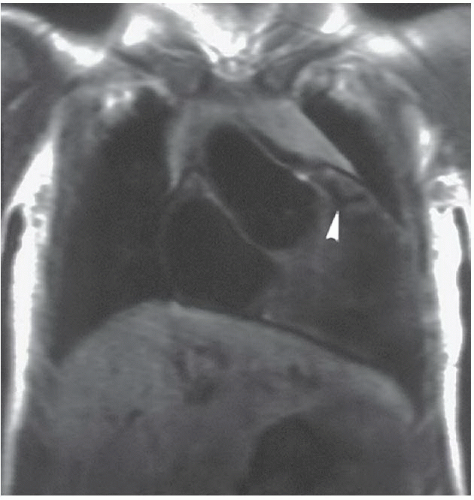
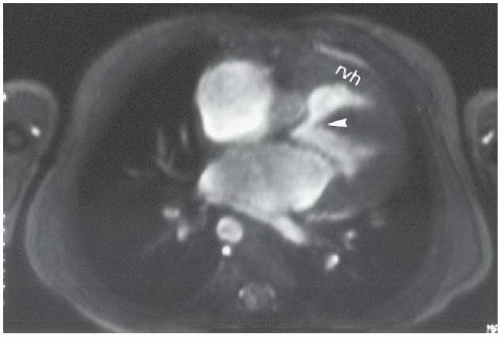
FINDINGS: The axial ECG-gated, T1-weighted MR image (Fig. 3.2.1) at the level of the great vessels demonstrates a right aortic arch (A). The coronal T1-weighted MR image demonstrates hypoplasia of the pulmonary infundibulum (Fig. 3.2.2, arrowhead). Axial gradient-recalled-echo MR images through the heart (Fig. 3.2.3) reveal right ventricular hypertrophy (rvh) and a high membranous ventricular septal defect (VSD) (arrowhead).
DIAGNOSIS: Tetralogy of Fallot
DISCUSSION: Tetralogy of Fallot is characterized by a combination of infundibular pulmonic hypoplasia, VSD, right ventricular hypertrophy, and overriding of the aortic root above the VSD (7). The pulmonic infundibular stenosis and overriding of the aorta facilitate shunting of deoxygenated blood from the right ventricle to the aorta, thereby producing cyanosis. Approximately one-fourth of patients with tetralogy of Fallot also have a right aortic arch, usually with mirror-image branching. The plain-film findings of right aortic arch with decreased pulmonary
vasculature should strongly suggest this diagnosis. These patients usually do not have cardiomegaly.
vasculature should strongly suggest this diagnosis. These patients usually do not have cardiomegaly.
MRI is often used to detect intracardiac and extracardiac abnormalities (8). In planning surgery for tetralogy of Fallot, it is important to assess the degree of hypoplasia of the pulmonary arteries and the development of collateral vessels from the systemic to pulmonary arteries. MRI analysis of measurements of the pulmonary artery and aortic root diameters has led to several methods for assisting in planning surgical repair and for predicting postoperative heart failure (91011). Surgical correction varies from palliative shunt procedures (e.g., Blalock-Taussig) to complete repair of the infundibular outflow obstruction and patching of the VSD (i.e., Lillehei procedure).
Aunt Minnie’s Pearls
Infundibular stenosis + VSD + overriding aorta + right ventricular hypertrophy = tetralogy of Fallot.
Right arch + decreased pulmonary flow + no cardiomegaly on radiographs = think tetralogy of Fallot.
Case 3.3
HISTORY: Two newborns with cyanosis
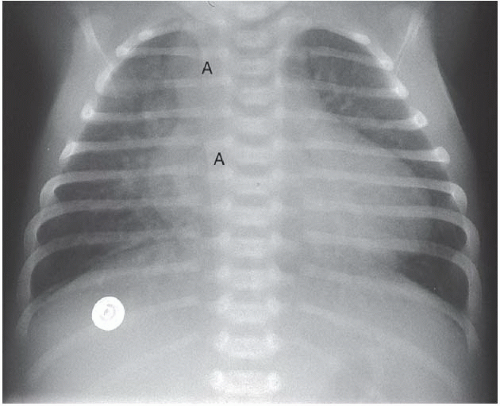
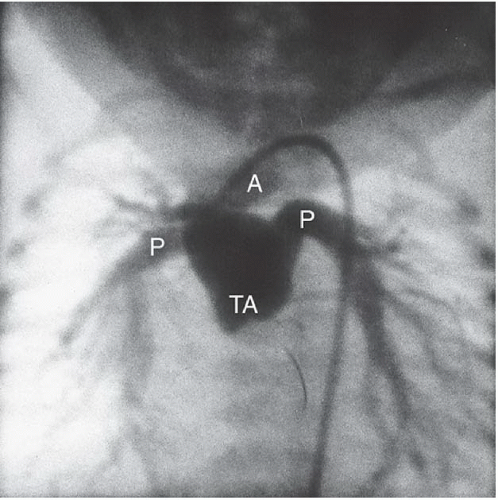
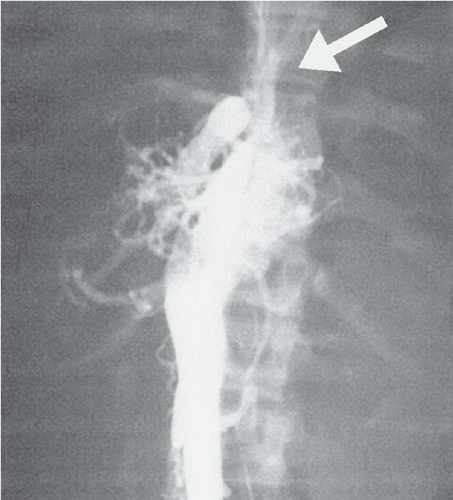
FINDINGS: A frontal chest radiograph demonstrates a right-sided aortic arch (A), cardiomegaly, and increased pulmonary blood flow (Fig. 3.3.1). An angiogram on another patient obtained by injection of contrast material into the aortic root (Fig. 3.3.2) reveals a single vascular trunk (TA), which gives rise to the aorta (A) and the pulmonary arteries (P).
DIAGNOSIS: Persistent truncus arteriosus
DISCUSSION: Between the fifth and seventh weeks of embryologic development, the aorta, pulmonary artery, and high ventricular septum are formed by fusion of the truncoconal ridges, which divide the truncus arteriosus into the aorta and pulmonary artery (12). Failure of the ridges to fuse causes a persistent truncus arteriosus and a defect in the ventricular septum. Radiographically, this diagnosis is strongly suggested when a right aortic arch (35% of cases), cardiomegaly, and increased pulmonary vascularity are present. Angiography or MRI can confirm the diagnosis by visualization of the persistent truncus. The truncal valve has two to five leaflets but is most commonly tricuspid (13). The most familiar classification scheme for persistent truncus arteriosus is the Collett and Edward’s system (14) that is based on the variable origin of the pulmonary arteries from the truncus. A main pulmonary artery arising from the left posterolateral aspect of the truncus (type 1) is the most common type. Surgical correction involves creating a new pulmonary outflow tract with synthetic graft material, although the truncal vessel becomes the aortic root. Before surgery, it is important to assess the position and origins of the coronary arteries on the imaging studies so that they are not inadvertently injured during the surgical procedure.
Aunt Minnie’s Pearls
Embryologic separation of the aorta from the pulmonary artery fails to occur in persistent truncus arteriosus.
Right arch + increased pulmonary flow + cyanosis = think persistent truncus arteriosus.
Case 3.4
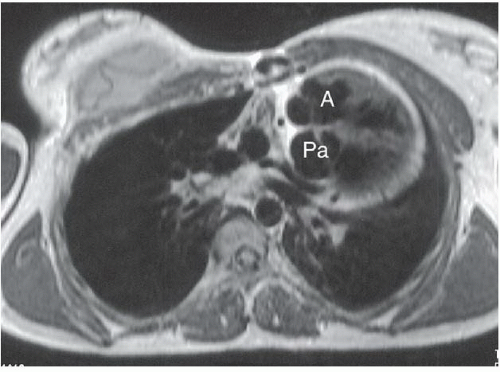
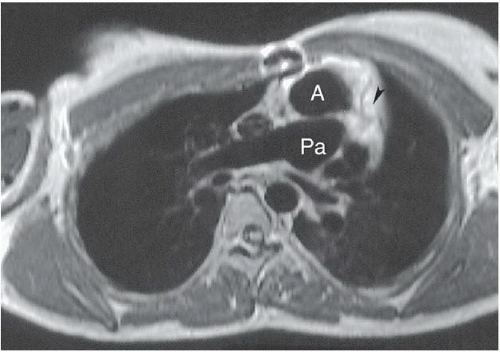
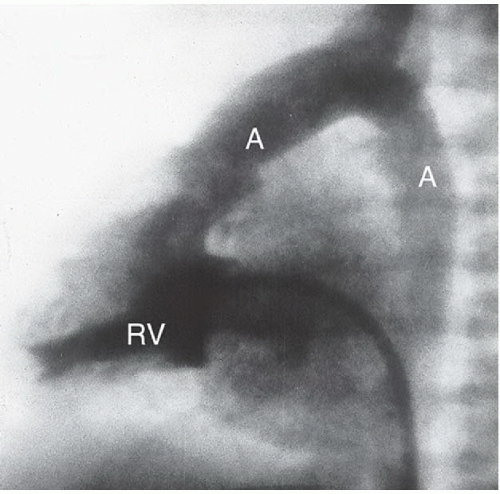
HISTORY: An 18-year-old woman with cyanosis as a newborn (Figs. 3.4.1 and 3.4.2). Figure 3.4.3 is of a different patient.
FINDINGS: Axial ECG-gated, spin-echo, T1-weighted MR images of the heart were obtained. The abnormal position of the aortic valve and aorta (A) anterior to the pulmonic valve and pulmonary artery (Pa) (Figs. 3.4.1 and 3.4.2) defines d-transposition. A coronary artery is seen originating from the aortic root (Fig. 3.4.2, arrowhead). In a different patient, a lateral view from an angiogram obtained with injection of contrast material into the right ventricle (RV) (Fig. 3.4.3) shows contrast opacifying the anteriorly displaced aorta (A).
DIAGNOSIS: d-transposition of the great vessels
DISCUSSION: Transposition of the great arteries is the most common congenital cyanotic heart lesion in the newborn. MRI allows a specific diagnosis of this lesion if the segmental method of cardiac analysis described by Van Praagh (15) is followed. In normal cardiac development, the inferior vena cava empties into a right-sided atrium, and the aortic valve is located posterior to and to the right of the pulmonic valve. This arrangement
describes situs solitus, normal aortic position, and a normal d-bulboventricular loop, respectively. The aorticopulmonary septum, which is responsible for dividing the truncus arteriosus into the two great vessels, normally undergoes a clockwise spiral. If this fails to occur, the aortic valve will lie anteriorly to the pulmonic valve, thereby defining transposition (16).
describes situs solitus, normal aortic position, and a normal d-bulboventricular loop, respectively. The aorticopulmonary septum, which is responsible for dividing the truncus arteriosus into the two great vessels, normally undergoes a clockwise spiral. If this fails to occur, the aortic valve will lie anteriorly to the pulmonic valve, thereby defining transposition (16).
A VSD is present in 40% of these patients, and valvular or subvalvular stenosis may occur in 30% of patients. Aortic transposition results in ventriculoarterial discordance (i.e., the right ventricle empties into the aorta). Atrioventricular concordance still exists in d-transposition; the venous blood travels from the right atrium to the right ventricle and out the aorta. An atrial septal defect (ASD), VSD, or patent ductus arteriosus is necessary to allow blood to mix between the systemic and pulmonary circulations.
Aunt Minnie’s Pearls
If the aortic valve is anterior to the pulmonic valve, transposition is present.
The dextro designation (d) indicates that the aortic valve is to the right of the pulmonic valve.
Surgical repair is necessary within 6 months to 1 year for optimal survival.
Case 3.5
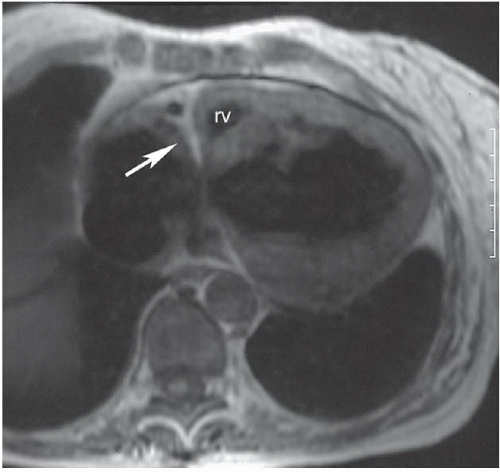
HISTORY: Young woman with severe cyanosis as a newborn
FINDINGS: ECG-gated, T1-weighted, spin-echo MR image reveals a linear bar of high signal intensity in the expected location of the tricuspid valve (Fig. 3.5.1, arrow). A large atrial septal artery on chest film = valvular pulmonic stenosis. An ASD (Fig. 3.5.2, arrowhead) and a large VSD (arrow) are present, as is an extremely hypoplastic right ventricle (rv) (Fig. 3.5.1).
DIAGNOSIS: Tricuspid atresia
DISCUSSION: When the tricuspid atrioventricular valve fails to develop normally, tricuspid atresia is present. The newborn presents with cyanosis, and radiographs classically demonstrate decreased pulmonary vascularity. MRI reveals a muscular and fatty ridge of tissue that separates a large right atrium from the hypoplastic right ventricle. A patent foramen ovale or ASD is necessary for blood to bypass the atretic valve and flow into the left atrium. The patient’s VSD allows a small amount of blood to pass into the pulmonary circulation through the hypoplastic right ventricle. Approximately 30% of patients with tricuspid atresia also have transposition of the great vessels (17), and pulmonic stenosis is also a common coexistent lesion. The presence of these associated lesions can have a great impact on the associated radiographic findings. Surgical correction involves palliative shunts to the pulmonary artery from the superior vena cava (Glenn) or right atrium (Fontan) and correction of the accompanying intracardiac shunts or transposition.
Aunt Minnie’s Pearls
Chest radiograph in tricuspic atresia shows decreased pulmonary vascularity.
MRI demonstrates a muscular and fatty ridge in the location of tricuspid valve and a hypoplastic right ventricle.
Tricuspid atresia is associated with an ASD and a VSD in all cases and transposition of the great vessels in 30% of patients.
Case 3.6
HISTORY: Mild cyanosis and a long systolic murmur identified on physical examination
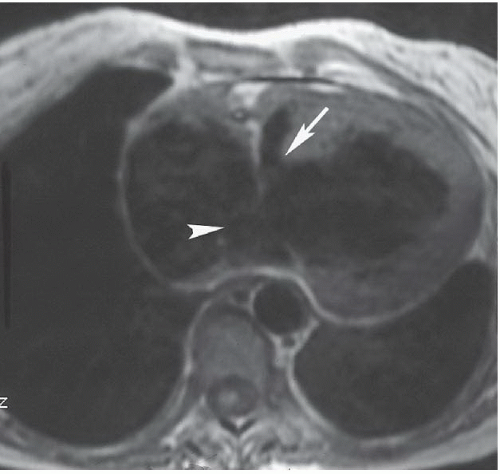
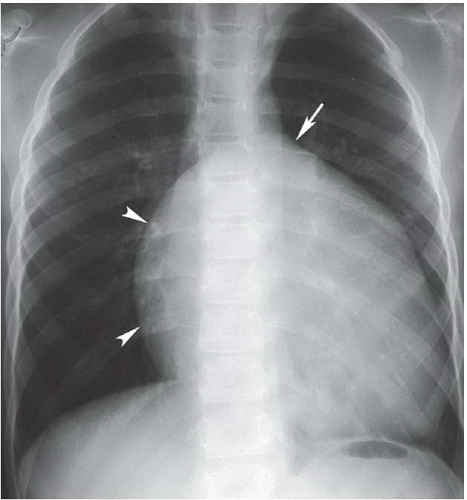
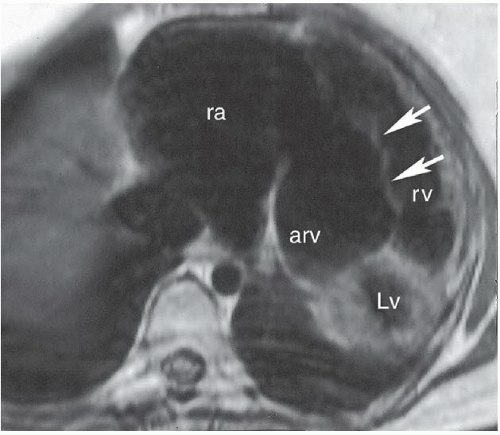
FINDINGS: Frontal view of the chest demonstrates an elongated and enlarged right atrium, which results in a box-shaped contour (Fig. 3.6.1, arrowheads). The pulmonary vascularity is decreased, and the area of the main pulmonary artery is concave on the frontal view (Fig. 3.6.1, arrow). In a different patient, an axial, ECG-gated, T1-weighted MR image of the heart reveals downward displacement and tethering of the tricuspid valve into the right ventricle (Fig. 3.6.2, arrows). This image also shows that a portion (arv) of the right ventricle (rv) is incorporated into the right atrium (ra). The left ventricle is also seen (Lv).
DIAGNOSIS: Ebstein anomaly
DISCUSSION: The Ebstein anomaly is abnormal downward displacement of the septal and posterior leaflets of the tricuspid valve into the right ventricle. As a result of this displacement, much of the right ventricle is anatomically incorporated into the right atrium, or “atrialized” (Fig. 3.6.2, arv). This portion of the ventricle has an abnormally thin wall, and tricuspid regurgitation occurs. An associated patent foramen ovale or secundum ASD is present in most cases. Radiographs may demonstrate a nearly pathognomonic appearance of an elongated and enlarged right atrium with a box-shaped contour, as seen in this case. Echocardiography, MRI, or angiography can confirm the diagnosis by demonstrating the displaced tricuspid leaflets and associated abnormalities. ECG abnormalities are frequently found and include right bundle branch block, prolonged PR intervals, and Wolff-Parkinson-White syndrome (18). An association has been described between this anomaly and the use of lithium in early pregnancy (19).
Aunt Minnie’s Pearls
Downward displacement of the septal and posterior leaflets of the tricuspid valve into the right ventricle indicates the Ebstein anomaly.
The Ebstein anomaly is associated with ECG conduction abnormalities and in utero lithium exposure.
Case 3.7
HISTORY: Acyanotic 11-year-old girl with upper-extremity hypertension
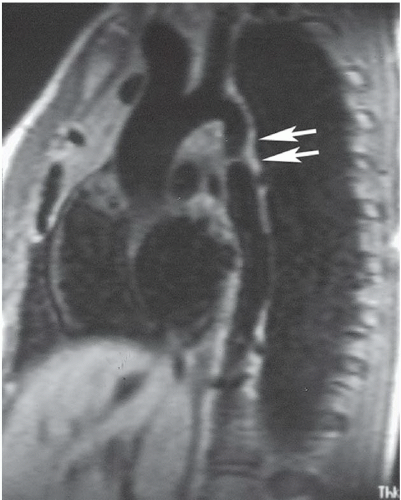
FINDINGS: Oblique sagittal, ECG-gated, T1-weighted MR image of the aorta demonstrates an area of abnormal narrowing in the region of the aortic isthmus (Fig. 3.7.1, arrows). Another image obtained off the midline reveals dilated, tortuous intercostal arterial collaterals (Fig. 3.7.2, arrows).
DIAGNOSIS: Coarctation of the aorta
DISCUSSION: The juxtaductal portion of the aorta is congenitally narrowed in coarctation of the aorta. If the narrowing occurs proximal to the ductus, blood is shunted to the descending thoracic aorta through the patent ductus. After the ductus closes, clinical symptoms may quickly develop with congestive heart failure and left-to-right shunting across a VSD. Postductal coarctations produce the more familiar presentation in which the radiographs demonstrate left ventricular hypertrophy, an indistinct aortic knob with a “three” contour, and bilateral rib notching. Coarctation of the aorta is associated with bicuspid aortic valve, VSD, patent ductus arteriosus, berry aneurysms, and Turner syndrome. Pseudo-coarctation refers to elongation of the thoracic aorta with kinking in the juxtaductal region, but no significant pressure gradient exists across the narrowing and no collateral vessels are present (20).
Surgical correction in patients younger than 10 years usually involves placement of a patch across the posterior aorta. Older patients, who are less subject to physical growth, are treated with a subclavian artery patch. Postsurgical restenosis is often treated with balloon dilatation.
MRI permits accurate follow-up evaluation after balloon angioplasty or surgical repair (21).
Aunt Minnie’s Pearls
Coarctation = juxtaductal narrowing of aortic arch with pressure gradient across the lesion (pseudo-coarctation = no pressure gradient).
This anomaly is frequently associated with bicuspid aortic valve, VSD, and patent ductus arteriosus.
Case 3.8
HISTORY: A 24-year-old woman with rapidly worsening ascites
FINDINGS: Anteroposterior (AP; Fig. 3.8.1) and oblique lateral (Fig. 3.8.2) inferior vena cava (IVC) cavagrams demonstrate stenosis of the suprahepatic IVC (arrow) and a spider-web appearance (arrowhead) of the hepatic vein elements.
DIAGNOSIS: Budd-Chiari syndrome
DISCUSSION: Hepatic venous obstruction (i.e., Budd-Chiari syndrome) is an uncommon cause of portal hypertension. Patients usually present with hepatosplenomegaly and rapid onset of ascites. Hepatic vein occlusion in adults can be the result of various hypercoagulable states (e.g., polycythemia vera or oral contraceptive use) or tumor invasion, as is seen in cases of hepatocellular or renal cell carcinoma. Often, a specific cause is not determined. Hemodynamic evaluation shows evidence of postsinusoidal venous obstruction, with elevated free and wedged hepatic vein pressures. The spider-web appearance of the wedged hepatic venogram is characteristic (22).
Aunt Minnie’s Pearls
A spider-web appearance of intrahepatic venous collaterals is characteristic of Budd-Chiari syndrome.
Hypercoagulable states and tumor invasion of the cava are common causes in adults.
Associated portal vein thrombosis may occur in 20% of patients with Budd-Chiari.
Case 3.9
HISTORY: An 8-year-old girl with hypertension and postprandial abdominal pain
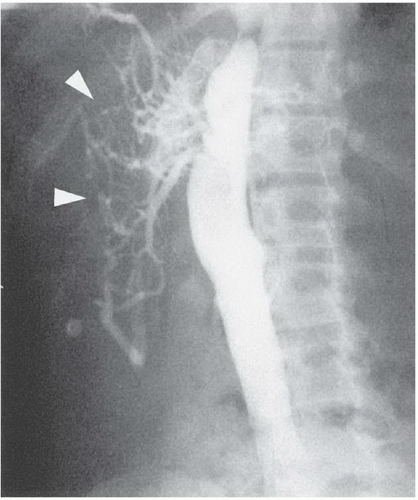
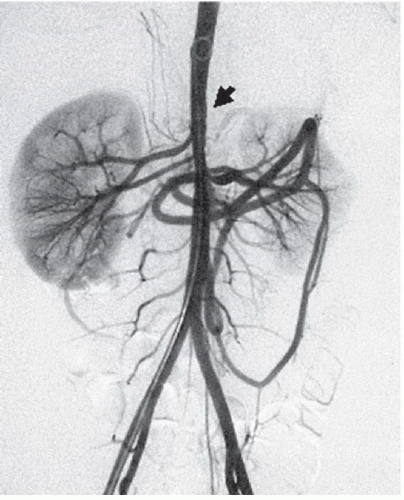
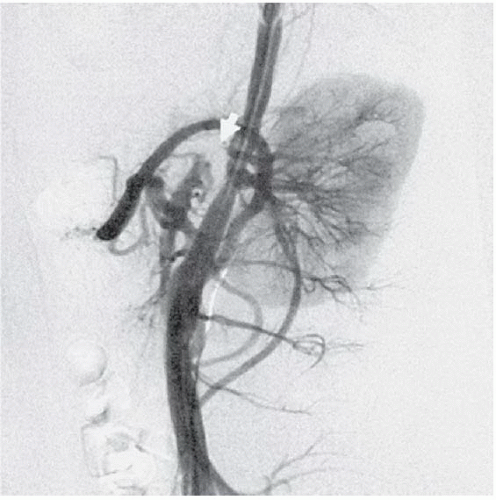
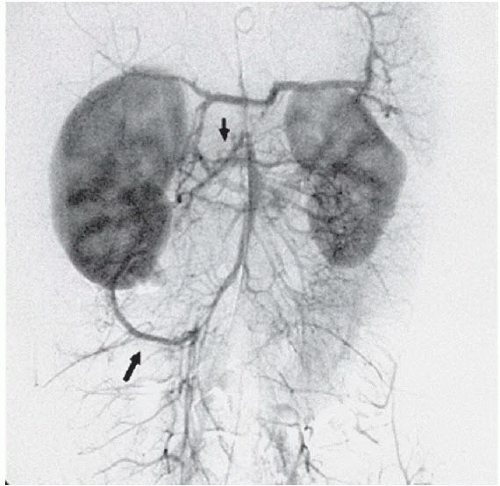
FINDINGS: Anteroposterior and lateral abdominal aortograms (Figs. 3.9.1 and 3.9.2) demonstrate occlusion of the celiac axis and superior mesenteric artery (arrows) and severe tapering of middle aorta. Late-phase image (Fig. 3.9.3) reveals reconstitution of these vessels through mesenteric collaterals (arrows).
DIAGNOSIS: Midaortic syndrome (MAS)
DISCUSSION: MAS is a rare vascular abnormality manifested by narrowing of the abdominal aorta and including the renal and visceral branches. This condition accounts for 0.5% to 2.0% of aortic coarctations and has also been referred to as coarctation of the abdominal aorta and subisthmic coarctation (232425). This is a potential cause of renovascular hypertension in children and adolescents, typically manifesting after the age of 5 years (26). MAS is a noninflammatory, nonatheromatous condition of unknown origin. The diagnosis is made with angiography. Angiography demonstrates smooth, segmental stenosis of the abdominal aorta, primarily involving the infrarenal aorta and bilateral proximal renal arteries. The disease may extend cephalad over time to involve the suprarenal aorta and the origin of the superior mesenteric artery. The inferior mesenteric artery is often spared (26).
Differential diagnosis considerations include Takayasu’s arteritis and neurofibromatosis. In Takayasu’s arteritis, the patients are older and present with systemic inflammatory signs and symptoms. Neurofibromatosis can have midabdominal aortic stenosis, but the diagnosis is usually known from other manifestations of the disease. Arterial bypass surgery is a highly successful treatment that can be curative. Balloon angioplasty can be used, but the results are often short term because of the progressive intimal and medial hyperplasia associated with the condition. Angioplasty may be useful for temporary relief of hypertension and as a bridge to surgery.
Aunt Minnie’s Pearls
MAS is a rare noninflammatory nonatheromatous vascular abnormality that accounts for 0.5% to 2% of aortic coarctations.
The cause is unknown.
Typical presentation is after the age of 5 years.
Case 3.10
HISTORY: A 28-year-old trauma patient with absent right lower-extremity pulses
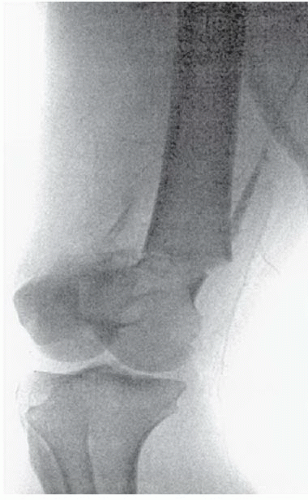
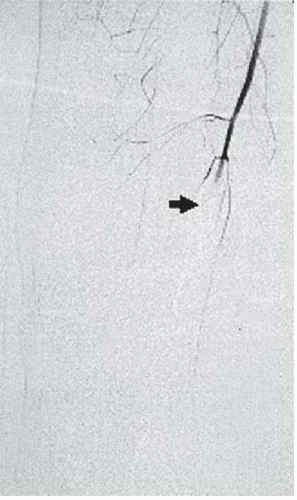
FINDINGS: Select right lower-extremity arteriogram shows a comminuted fracture of the distal femur (Fig. 3.10.1) with occlusion of the popliteal artery (Fig. 3.10.2, arrow). There is a trace amount of blood flow below the knee (Fig. 3.10.3, open arrow).
DIAGNOSIS: Popliteal artery occlusion (traumatic)
DISCUSSION: Injury to the popliteal artery accounts for 5% to 19% of civilian arterial injuries and results in more amputations than any other vascular injury (27,28). Intimal injury, associated with thrombosis and transection, occurs more often with blunt than penetrating trauma. Knee dislocations are the most common type of associated musculoskeletal injury (29). Because of its course, the popliteal artery is anatomically vulnerable to trauma. The artery is tethered between the tendinous arch of adductor magnus and soleus muscle, rendering it susceptible to stretch injuries and unprotected from direct trauma. Popliteal artery thrombosis can also be complicated by venous injury and compartment syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree