Carotid ultrasound is the primary noninvasive method for detecting, grading, and monitoring internal carotid artery (ICA) stenosis. Major components of carotid Doppler ultrasound include assessment of ICA stenosis using Doppler velocity criteria, spectral waveform analysis, and assessment of ICA stenosis. Recently, the Intersocietal Accreditation Commission Vascular Testing put forth new modified Society of Radiologists in Ultrasound criteria with a higher peak systolic velocity threshold of 180 cm/s for 50% to 69% ICA stenosis. Additional emerging techniques, including 3D imaging, contrast-enhanced ultrasound, and superb microvascular imaging, may help identify vulnerable plaque and thus help further risk-stratify patients in the future.
Key points
- •
Most strokes are ischemic, with approximately 15% of embolic strokes originating from vulnerable plaque at the carotid bifurcation.
- •
Carotid ultrasound (US) is the primary noninvasive method for detecting, grading, and monitoring internal carotid artery (ICA) stenosis.
- •
The Intersocietal Accreditation Commission Vascular Testing now recommends a modified Society of Radiologists in Ultrasound criteria peak systolic velocity threshold of 180 to 230 cm/s for the diagnosis of 50% to 69% ICA stenosis in the absence of an ICA/CCA ratio greater than 2.0. Peak systolic velocities greater than 230 cm/s remain the primary Doppler US criteria for diagnosing ≥70% stenosis. However, findings should be correlated with grayscale and color Doppler findings as well as secondary parameters, including peak systolic ratio and end-diastolic velocity.
- •
Carotid spectral waveforms may help diagnose cardiovascular disease as well as nonatherosclerotic pathologic conditions.
Introduction
In the United States, cerebrovascular disease is a significant cause of morbidity, with stroke being the fifth leading cause of death. In 2020, someone died of stroke every 3.3 minutes on average, and the direct and indirect cost of stroke in the United States was estimated at $56.5 billion. Risk factors for stroke include hypertension, obesity, hyperglycemia, hyperlipidemia, renal dysfunction, and atrial fibrillation, among others.
Approximately 87% of all strokes are ischemic. Significant carotid stenosis is found in 10% to 20% of patients with transient ischemic attack (TIA) or stroke. Most ischemic strokes occur because of atheromatous emboli, with approximately 15% of embolic strokes originating from plaque at the carotid bifurcation. The remaining 13% of all strokes are hemorrhagic, most which are related to hypertension.
In 2002, the Society of Radiologists in Ultrasound (SRU) published specific recommendations on the ultrasound (US) evaluation of carotid artery disease. These criteria were based on a set of Doppler velocity criteria aimed at grading the severity of internal carotid artery (ICA) stenosis according to a method adopted by the North American Symptomatic Carotid Endarterectomy Trial (NASCET). , As a result, Doppler US has become the primary noninvasive method for detecting, grading, and monitoring ICA stenosis owing to its high sensitivity and specificity, lack of ionizing radiation, widespread availability, and low cost. Carotid Doppler US involves 3 major components: plaque characterization, assessment of ICA stenosis using Doppler velocity criteria, and spectral waveform analysis.
Technique
Carotid US is typically performed in the supine position with the patient’s neck slightly hyperextended and turned toward the contralateral side using a high-resolution 5-MHz or greater linear-array transducer. Linear transducers in the 5- to 12-MHz range or 2- to 9-MHz range are often used. The examination should include real-time scanning with grayscale, color Doppler, and spectral Doppler imaging. A 3- to 5-MHz curved array transducer may also be used in patients with a large body habitus or in patients with deep or densely calcified vessels. The vessels can be imaged from an anterior or posterior approach; however, the sternocleidomastoid muscle often provides a good acoustic window. Angulation of the transducer caudally in the supraclavicular area and cephalad at the level of the mandible can also aid visualization.
Carotid US should routinely include assessment of the proximal and distal common carotid arteries (CCAs); the carotid bifurcation; the proximal, mid, and distal internal carotid arteries (ICAs); the proximal external carotid arteries (ECAs); and the mid–vertebral arteries (VAs) in the sagittal and transverse planes using grayscale and color Doppler imaging. In addition, dedicated images of any areas of suspected stenosis or other pathologic condition should also be acquired.
On grayscale imaging, gain is optimized for the assessment of plaque, the vessel wall (such that the normal 3 layers of the CCA wall are clearly depicted), and any other noted abnormalities. When manually placed, the focal zone is placed at the posterior margin of the vessel. Color Doppler is used to identify areas of luminal narrowing or abnormal flow to select regions for spectral analysis. The color gain is adjusted so that color signal remains within the vessel lumen. If no flow is seen, increasing the color gain, decreasing the wall filter, decreasing the color scale, or using power Doppler imaging can improve sensitivity for the detection of flow, particularly in arteries in which near complete occlusion is suspected.
Long-axis spectral Doppler velocity measurements should be obtained at the predetermined sites in all vessels as well as in areas of stenosis or suspected stenosis. If a significant stenosis is found or suspected, images must be recorded at the location of the stenosis with the maximum velocity as well as distal to the site of the maximum velocity to document the presence or absence of poststenotic turbulent flow. The Doppler angle should be between 45° and 60°. The potential velocity error associated with Doppler angle increases with increasing Doppler angle, particularly at a Doppler angle greater than 60°. For this reason, Doppler angles greater than 60° should be avoided whenever possible. The peak systolic velocity ratio (PSVR) is calculated by dividing the peak systolic velocity (PSV) at or just distal to the ICA stenosis by the PSV in the distal CCA. Three measurements of the PSV and end-diastolic velocity (EDV) should be recorded at each vessel. The PSV in the distal CCA should be measured 2 to 3 cm below the carotid bulb where the walls are parallel to one another. For spectral Doppler, the sample gate is placed centrally within the lumen and kept between 1.5 and 2.5 mm in width. The Doppler scale or pulse repetition frequency should be set to maximize the size of the waveforms without aliasing.
Normal findings
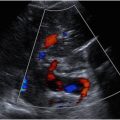
Typically, the right CCA arises from the brachiocephalic artery, and the left CCA arises directly off the aorta. Many vascular variants exist, including a common origin of the left brachiocephalic artery and the left CCA (bovine arch), which occurs in approximately 15% of the population. Near the carotid bifurcation, the distal CCA caliber increases slightly to form the carotid bulb. The CCA then bifurcates into its 2 terminal branches, the ICA and ECA. The ICAs supply oxygenated blood to the anterior circulation of the brain, and the ECAs supply the structures of the head, face, and neck. The ECA is most reliably distinguished from the ICA by its branches in the neck ( Fig. 1 ) because the ICA does not have extracranial branches. The ICA is also typically larger and located posterolateral relative to the ECA, although these are not reliable distinguishing features because of significant anatomic variation.

The CCA, ICA, and ECA have unique waveform patterns that are related to differences in the tissues they supply. The carotid arteries should demonstrate a sharp systolic upstroke and narrow spectral envelope outlining a spectral window. The ICA typically has a thicker spectral envelope and continuous diastolic flow owing to lower peripheral vascular resistance and higher oxygen consumption of the brain. The ECA waveform typically has a relatively thinner spectral envelope and less diastolic flow owing to higher peripheral vascular resistance and lower oxygen consumption in the head and neck musculature. Because of variable oxygen consumption in the head and neck vasculature, the amount of diastolic flow of the ECA can vary; however, the amount of diastolic flow should be symmetric bilaterally and should be less than the ICA. The CCA has a waveform morphology between that of the ICA and ECA, with PSV ranging from 60 to 100 cm/s and continuous diastolic flow.
Plaque characterization
Plaque characterization is a vital component of the assessment of carotid artery US. The risk of plaque embolization is directly related to the plaque composition and morphology ( Fig. 2 ). Factors that predispose to plaque rupture include intraplaque hemorrhage, necrosis, inflammation, and thinning of the fibrous cap, which results in the formation of unstable thrombus on the plaque surface. The unstable thrombus may then embolize as it is dislodged by the high-velocity blood coursing through the narrowed lumen. As a result, the degree of stenosis is also indirectly related to the risk of embolization and stroke. Ulcerated plaque is also at increased risk of distal embolization owing to exposure of the thrombogenic centrally necrotic core or stagnant flow. Smoothly surfaced hyalinized, fibrous, or calcified plaque has a lower risk of rupture and distal embolization because of a lack of unstable thrombus on the plaque surface and lack of exposure of the centrally necrotic core.

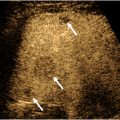
Plaque burden, echogenicity, and surface characteristics should be assessed using grayscale and color Doppler. Plaque may be described as either hypoechoic or hyperechoic (echogenic), and its composition may be homogeneous, mixed, or heterogeneous. In addition, the plaque surface should be examined and may be either smooth or irregularly surfaced/fissured. Ulcerated plaque is defined as a surface defect greater than 2 mm identified in 2 orthogonal imaging planes and is at increased risk of plaque rupture. Hypoechoic plaque is more likely to be lipid-laden or hemorrhagic and is more likely to rupture. As a result, heterogeneous plaque is generally regarded as unstable, as it contains hypoechoic and/or anechoic areas, which indicate intraplaque cholesterol or hemorrhage ( Fig. 3 ). Conversely, echogenic plaque is generally regarded as stable and is unlikely to rupture.

Optimizing carotid US scanning technique is vital in plaque characterization. Plaque may appear falsely hypoechoic or falsely echogenic if the grayscale gain is set incorrectly. Appropriately setting the color gain is also important to avoid obscuring hypoechoic plaque or thrombus, including markedly hypoechoic plaque or thrombus, which may only be seen as a color void on Doppler imaging and not readily detectable on grayscale imaging.
Intima-media thickness (IMT) is an additional parameter that has been proposed as an independent risk factor for cardiovascular disease and a predictor of stroke. In 2008, the American Society of Echocardiography issued a consensus statement recommending that the IMT be measured on magnified images along the posterior wall of the distal CCA 1 cm below the bulb using a high-frequency linear-array transducer with the CCA oriented perpendicular to the US beam, avoiding areas of plaque and ensuring not to include the echogenic adventitia in the measurement. An IMT measurement less than 0.7 to 0.8 mm is considered normal, although IMT does vary with age, gender, and ethnicity. Although previous studies have suggested that IMT is an independent risk factor for cardiovascular disease, more recent studies suggest that carotid IMT measurement does not provide meaningful risk stratification for future cardiovascular events. , A recent international consensus statement defines carotid plaque as diffuse IMT thickening greater than 1.5 mm or any focal protuberant plaque, and repeated follow-up measurements are recommended in these patients even in the absence of symptoms.
Three-dimensional (3D) imaging of atherosclerotic plaque has also been reported to be highly accurate and a predictor of increased risk of cardiovascular events. Given that atherosclerotic plaque is a 3D phenomenon, recent studies have suggested that 3D quantification of carotid plaque is more sensitive in the detection of clinically significant stenoses as compared with 2-dimensional imaging. Patients in the top quartile of total plaque area on 3D imaging have also been shown to have a 3 times higher 5-year risk of death, stroke, or myocardial infarction. , An additional advantage of 3D imaging is an improved ability to evaluate the plaque surface. Patients with a global ulcer volume greater than 5 mm 3 were shown to have a considerably increased risk of stroke, TIA, or death.
Contrast-enhanced ultrasound (CEUS) is an additional technique, which has been shown to be superior to color Doppler imaging in terms of grading stenosis and assessing the plaque surface. , As US contrast agents are purely intravascular agents, CEUS is also a valuable method for the detection of intraplaque neovascularization, a major feature of plaque vulnerability. , Delayed phase imaging has also shown increased enhancement of symptomatic plaques relative to asymptomatic plaques, a sign of intraplaque inflammation. However, although CEUS has shown utility in identifying vulnerable plaque and neovascularization, standardized CEUS technique has not yet been established. , Nevertheless, CEUS could be incorporated into future risk stratification systems for patients with carotid plaques, particularly those with 50% to 69% stenosis. , , Superb microvascular imaging (SMI) is an additional novel US technique, which applies an algorithm to differentiate true microvascular flow signals from wall motion artifacts and clutter. Several studies have suggested SMI as a promising noninvasive technique, which can detect neovascularity and assess plaque stability with accuracy comparable to CEUS. ,
Internal carotid artery stenosis grading
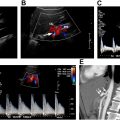
In 1991, the NASCET reported that carotid endarterectomy (CEA) was highly beneficial in symptomatic patients with recent hemispheric or retinal TIAs or nondisabling strokes and ipsilateral high-grade ICA stenosis (70%–99%), showing an absolute ipsilateral stroke risk reduction of 17% compared with medical treatment. In the NASCET trial, the percent ICA stenosis was calculated by dividing the narrowest luminal ICA diameter by the luminal diameter of the distal normal ICA ( Fig. 4 ). This method has now become widely accepted as the preferred method of grading ICA stenosis, although plaque burden can be significantly underestimated using this method, particularly when there is decreased flow in the poststenotic ICA owing to collateral vessels or tandem lesions. Additional trials also showed a significant but smaller benefit in asymptomatic patients with moderate ICA stenosis (50%–69%). , Subsequent trials also showed a similar decrease in ipsilateral stroke incidence following carotid artery stent placement. ,

In 2002, the SRU consensus panel recommended using PSV and the presence of plaque as the primary Doppler criteria for grading ICA stenosis. PSVR and EDV serve as secondary parameters with grayscale and color Doppler correlation. , Since then, the SRU consensus guidelines have become the most commonly used criteria for assessing ICA stenosis in the United States.
Updates
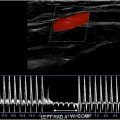
In 2014, the Intersocietal Accreditation Commission (IAC) Vascular Testing issued a White Paper detailing significant variability in the reporting of carotid artery stenosis as well as the potential clinical impact on this variation. The IAC then carried out a research study aimed at assessing validity of the SRU Consensus criteria in an effort to potentially refine the criteria. In this study, the primary parameter for ≥50% ICA stenosis of PSV of ≥125 cm/s did not meet prespecified thresholds for adequate sensitivity, specificity, and accuracy (sensitivity, 97.8%; specificity, 64.2%; accuracy, 74.5%). Test performance was improved by raising the PSV threshold to ≥180 cm/s (sensitivity, 93.3%; specificity, 81.6%; accuracy, 85.2%). These data supported the adoption of a higher PSV threshold of 180 cm/s for 50% ICA stenosis and found the previously used threshold of 125 cm/s to be too sensitive with insufficient specificity and accuracy. As a result, the IAC Vascular Testing now recommends the adoption of modified SRU criteria with a higher PSV threshold value of 180 cm/s for 50% ICA stenosis ( Fig. 5 , Table 1 ). However, the IAC continues to recommend correlation with grayscale and color Doppler findings, as there are instances of 50% to 69% ICA stenosis with PSV of ≥125 and less than 180 cm/s. In these cases, the images should demonstrate other sonographic features of stenosis, such as an elevated ICA/CCA PSV ratio greater than 2.0, significant atherosclerotic plaque, and color aliasing at the site of stenosis ( Fig. 6 ). In the event of significant discrepancies between the spectral Doppler and the grayscale and color Doppler findings, an explanation should be sought factoring in individual patient factors, such as the patient’s hemodynamics or other factors affecting physiology. If no clinical explanation is evident, additional imaging with computed tomographic (CT) angiography may be of benefit. Although IAC Vascular Testing is now recommending a higher PSV threshold of 180 cm/s for 50% to 69% stenosis, there was insufficient evidence to recommend modification of the SRU Criteria for higher degrees of stenosis (≥70% ICA stenosis) owing to a limited sample size ( Fig. 7 ). However, combinations of PSV ≥230, ≥250, and ≥260 cm/s with ICA/CCA ratio ≥3.3 all met criteria for adequate sensitivity, specificity, and accuracy.