Central venous occlusions (CVOs) of the systemic circulation are highly morbid, causing significant symptoms from venous congestion, venous thromboembolism (VTE) and impaired vascular access. Endovascular recanalization (EVR) has emerged as the treatment of choice for medically refractory nonthrombotic and thrombotic CVOs with Intravascular Ultrasound (IVUS) playing a pivotal role. Radial and side-firing IVUS catheters are used during central venous recanalization in the chest, abdomen and pelvis. The intraluminal ultrasonic view of a CVO shows dynamic details of pathology not obtained with conventional venography or cone-beam CT, allowing for a more accurate evaluation of clot burden, wall integrity, tumor invasion, occlusion length and luminal caliber. IVUS is also superb for guiding treatment of CVOs involving blunt and sharp recanalization, stenting, and mechanical thromboembolectomy (MTE) of VTE, especially high-risk free-floating thrombi (FFT) and clot in-transit (CIT) in the right atrium (RA). It most accurately assesses procedural endpoints, including successful intravascular traversal across an occlusion, adequate luminal gain after venoplasty and stenting, and complete clot extraction during thrombectomy. Moreover, this is all done without added contrast or radiation, which is paramount to reduce exposure during a challenging recanalization, especially in the aging hemodialysis population. Long-term data is now available that shows IVUS can enhance technical and clinical success and reduce complications during EVR. To achieve these benefits with IVUS interventionalists must be familiar with the available catheters and how to optimize and interpret the intraluminal images obtained. This paper will review the patient evaluation, indications, equipment, steps, challenges, complications, and outcomes for central venous recanalization (CVR) and RA thrombectomy with IVUS.
Clinical Evaluation and Indications
Fundamentals
A systemic CVO is a complete blockage of in-line flow in a large systemic venous conduit of the central body cavities (chest, abdomen or pelvis). The transition to a central venous branch in the upper and lower body occurs at the thoracic outlet in the chest and inguinal ligament in the abdomen and pelvis. The superior and inferior vena cava (SVC and IVC) are the most central and sizeable veins of the systemic circulation above and below the diaphragm. These sustain venous return into the right atrium (RA) alongside the pulmonary circulation to the left atrium. The cavae and adjacent major central systemic veins make up the outflow for visceral, superficial and deep systemic branches of the head and neck (H&N), peripheral extremities, and trunk. Branches from the upper body ultimately converge into the Superior Innominocaval (SIC) system in the thorax and from the lower body into the Inferior Iliocaval (IIC) system in the abdomen and pelvis ( Table 1 ). The portal system is a unique somewhat autonomous collection of visceral drainage, including the gastrointestinal, pancreaticobiliary and splenic veins which conventionally flow into the liver prior to emptying into the IIC via the hepatic veins. CVOs in each distribution have specific etiologies and regional manifestations that dictate the presentation, diagnosis, and management. Notable examples are SVC syndrome (SIC), lower extremity venous insufficiency (IIC) and Budd-Chiari with portal hypertension (hepatoportal system and IIC). An especially concerning scenario that affects both distributions is FFT and CIT in the RA. These pose substantial morbidity and mortality with the most feared sequela being a massive pulmonary embolus. In select patients endovascular intervention using large bore aspiration catheters under IVUS guidance has been shown to be safe and effective for RA thrombectomy. This paper will review the use of IVUS in CVR for occlusions in the SIC and IIC distributions and briefly touch upon the portal system and RA thrombectomy. Before covering the technical aspects of IVUS, it is necessary to review relevant pathophysiology and anatomy.
Pathophysiology
Venous occlusions manifest from three basic mechanical processes :
- 1.
Intraluminal obstruction from thrombus, hyperplasia, devices, or tumors.
- 2.
Extraluminal compression from a viscera, vessel, mass, collection, hardware, or musculoskeletal tissue causing intermittent or fixed venous effacement.
- 3.
Stricture formation from intrinsic vein wall injury resulting in reactive fibrotic wall thickening, contracture, and eventual obliteration of the lumen.
The underlying pathologies that drive an occlusion are broadly divided into benign or malignant etiologies, which portend different treatment approaches and prognosis. The most frequent benign pathologies are hemodialysis related complications, provoked and unprovoked deep venous thrombosis (DVT), device related thrombophlebitis with fibrosis (catheters, IVC filters, and cardiac leads), and compression syndromes (Thoracic outlet, Nutcracker, and May-Thurner). Common malignant CVOs come from invasion and/or compression by lung cancer (NSCLC), lymphoma, thymic cancer, liver tumors (HCC), renal cancers (RCC), adrenal carcinoma, germ cell tumors, leiomyosarcoma and gynecologic tumors. Chronic CVOs often involve all three elements since etiologies that cause intraluminal obstruction and compression lead to strictures and vice versa. For example, a compressive May-Thurner that causes an occlusive stricture in the left common iliac vein with DVT extending peripherally from the occlusion into the ipsilateral extremity veins. It is important to note that DVTs are both a cause and effect of benign and malignant CVOs due to stimulation of Virchow’s triad (stasis, injury and hypercoagulability).
Anatomy
Superior Innominocaval (SIC)
Conventially, the SIC distribution consists of the SVC, Azygous vein (AzV), paired Innominate veins (INV), paired Subclavian veins (SbV) and paired Internal Jugular veins (IjV). These make up the major central venous conduits of the H&N, upper extremities (UEXT) and chest cavity bilaterally via the following in-line paths ( Table 1 ):
- •
H&N: IjV > INV > SVC > RA
- •
UEXT: SbV > INV > SVC > RA
- •
Chest cavity: AzV > SVC > RA
The adult SVC is a 7 cm long valveless compliant vein in the right posterior mediastinum. It spans from the INV confluence at the level of the right 1st costal cartilage to the superior cavoatrial junction (CA) at the 3rd costal cartilage, approximately 2 vertebral bodies below the carina on an anterior-posterior radiograph. The reported average adult diameter of the SVC by CT evaluation is 2.1 cm, but ranges from 1.5 to 2.8 cm due to flaring at the junction points. The AzV forms an arch that inserts posteriorly into the SVC just above the right mainstem bronchus. The infra-azygous SVC enters the fibrous pericardium before draining into the right atrium via the sinus venarum posteriorly, resulting in a very different presentation and treatment approach than the supra-azygous segment along the right parietal pleura. The paired valveless INVs conjoin into the SVC above the azygous with an average adult diameter of 1.4 cm (range of 1.0-1.8 cm) and length of 6 cm on the left due to crossing the arch and 3 cm on the right. The IjVs and SbVs converge to form the INVs bilaterally, draining the head and neck and upper extremities respectively. The IjV is valved and spans from the jugular sinus to the INV coursing anterolateral to the carotid artery within the carotid sheath and posterior to the clavicle before entering the thorax. The average diameter ranges from 1.4 to 1.7 cm with the right IJV slightly larger than the left. The SbVs are often valveless coursing below the clavicle and anterior to the subclavian artery from the lateral margin of the first rib and clavicle (thoracic outlet) to the confluence with the IjV at the sternoclavicular joint along the medial border of the anterior scalene muscle. The left ScV is longer and drains the thoracic duct at the venous angle with the IjV whereas the right drains the right lymphatic duct. The average diameter is around 1.2 cm in the neutral position, but ranges from 0.8 to 1.6 cm with varying degrees of abduction and adduction at the thoracic outlet. Noteworthy venous branches that drain into these major central SIC veins include the external jugular, vertebral, inferior thyroidal, thymic, thyrocervical, costocervical, internal mammary, supreme intercostal, internal thoracic, pericardiophrenic, hemiazygous and accessory hemiazygous veins. These all serve as potential points of collateral drainage to bypass a CVO in the SIC and maintain venous return to the right heart. Notable congenital variants of a persistent left-sided SVC and partial anomalous pulmonary venous return are also important to consider.
Inferior Iliocaval (IIC)
The central veins of the IIC distribution are defined as the IVC and Iliac veins along with major visceral branches, including the hepatic veins (HV), renal veins (RV), gonadal veins (GV) and portal system (PS), which drain via the liver into the HVs. Conventionally, these drain the lower extremities and abdominopelvic viscera via the following in-line paths ( Table 1 ):
- •
Lower extremities (LEXT)- External iliac vein (EIV) > Common iliac vein (CIV) > IVC
- •
Pelvis- Internal iliac vein (IIV) > CIV > IVC
- •
Hepatoportal (HP)- Mesenteric veins (SMV and IMV) + Splenic vein (SV) + Pancreaticoduodenal veins (PDV) + Gastric veins (GV) > Portal veins (PV) > Liver > Hepatic veins (HV) > IVC
- •
Right renal (RRV) and gonadal (RGon) veins > IVC vs Left gonadal (LGon) and adrenophrenic > left renal vein (LRV) > IVC
The bilateral CIV and IVC form the Y-shaped bottleneck that all pelvic and lower extremity veins converge towards. The IVC is a valveless vein residing in the right retroperitoneal space from the common iliac confluence at L5 to the inferior cavoatrial junction across the central tendon of the diaphragm at T8. The average diameter in most adults is around 1.9 cm in males and 1.7 cm in females with a range from 1.2 to 2.8 cm. The upper limit of caliber is most often noted in the suprarenal IVC where the vein dilates after the renal confluence. When the IVC is larger than 2.8 cm it is deemed a Megacava, which is important when considering sizing of stents and filters. The IVC is divided into the infrarenal, juxtarenal and suprarenal segments based on the insertion of the renal veins at L1 and the intrahepatic and suprahepatic segments relative to the hepatic venous confluence at T8. The common, external and internal iliac veins are also valveless with the common iliac having an average diameter of 1.6 cm on the left and 1.4 cm on the right, the external iliac measuring around 1.2-1.4 cm, and the internal iliac measuring around 0.5-1.0 cm. The left common iliac vein is generally longer and more diagonally oriented relative to the right common iliac given it crosses midline to reach the IVC confluence. Along this crossing point the vein can be compressed between the contralateral right common iliac artery and the lumber spine at L5 (May-Thurner physiology) with resultant stricture or web formation in the setting of recurrent pulsatile injury. Additional smaller noteworthy venous branches of the IIC include the ascending lumbar, vertebral plexus (Batson plexus), obturator, corona mortis, inferior epigastric, middle rectal, inferior rectal, plexiform retroperitoneal veins (veins of Retzius), inferior phrenic, and hemiazygous veins. Common portosystemic anastomoses that bypass the liver include the gastric veins to azygous vein (left, posterior and short gastrics), a splenorenal shunt, a gastrorenal shunt (posterior gastric to left renal vein via the adrenophrenic trunk), paraumbilical to epigastric veins and superior rectal to middle/inferior rectal veins. These all serve as key points of collateralization for CVO of the IIC veins and PS. Notable congenital variants are a left IVC, duplicated IVC, agenesis of the intrahepatic IVC, anomalous continuation of the IVC into the azygous, IVC webs, Absent IVC and Abernethy malformations.
Venous Variations
Inherent anatomic differences and physiological effects are known to influence the normal caliber, flow, and appearance of central systemic veins on imaging. Anatomic differences are static variations between patients with the following factors reported in the imaging literature:
- •
Gender: Male veins are thought to be 1-2 mm larger in average diameter, however conflicting CT based data shows the IIC veins are up to 2 mm larger in females of equivalent size. Also, the gonadal and pelvic veins differ in women and lend to higher rates of pelvic congestion and lower extremity venous insufficiency related to pregnancy.
- •
Body Habitus and Age: A positive linear correlation is noted with Height and Weight for larger diameter and length, but the correlation with Age is less clear.
- •
Morphology: Venous flaring at junctions and confluences (ex. cavoatrial junction, supra-azygous SVC and suprarenal IVC) results in nonuniform diameters.
- •
Laterality: Vein diameter is greater on the Right by approximately 1-2 mm and has potential correlation with limb dominance, but length and angulation are greater on the left given crossover to right-sided cavae and azygous veins (ex. left innominate, renal, hemiazygous, lumbar and iliac veins). Left tributaries also differ, such as the gonadal and adrenophrenic veins which drain to the renal vein on the left and IVC on the right.
- •
Congenital variants
- ○
Fenestration- Short segment division of a branch with reconvergence before terminal drainage
- ○
Duplication- Separate branches with distinct origins and/or terminal drainage (partial or complete)
- ○
Situs reversal (ex. Left SVC or IVC with or without congenital heart disease)
- ○
Interruption, agenesis, or absences of a segment/branch (ex. azygous continuation of the IVC)
- ○
Compression syndromes (ex. Paget-Schroetter, Nutcracker, May-Thurner and Popliteal entrapment)
- ○
Dynamic physiological variations can continuously or transiently influence the appearance and measurement of normal and abnormal veins on imaging as well. This includes:
- •
Volume status- Diameter shows a significant positive linear correlation only when extreme volume shifts are reached (ex. hemorrhagic shock or cardiac/renal overload), but otherwise the majority of volume shifts cause < 1 mm difference (eg, NPO for sedation)
- •
Cardiac cycle- End systolic > End diastolic diameter by 1-4 mm
- •
Inflow: Increased with visceral function (ex. postprandial portomesenteric flow and postexercise skeletal vasodilation) and Hemodialysis arteriovenous fistula
- •
Position
- ○
Abduction and flexion of the shoulder (compress subclavian vein) and hip (compress common femoral vein)
- ○
Prone (Multifactorial, but favored to increase venous return)
- ○
Trendelenburg (Upper > Lower vein distension) and Reverse Trendelenburg (Lower > Upper vein distension)
- ○
Ipsilateral neck rotation (compress internal jugular vein)
- ○
- •
Respiratory phase: Expiratory venous pressure and diameter > inspiratory
- •
Pregnancy: Fetal circulation increases venous volume and diameters, and mass effect compresses IIC veins.
Knowing the pathophysiology, anatomy and variations of veins, particularly the normal course and caliber, is essential to using IVUS successfully during CVR. , ,
Clinical Presentation and Indications
CVOs can present with a wide range of clinical findings. The most common symptoms are from subacute to chronic venous congestion downstream of the occlusion. However, more worrisome findings of organ dysfunction do occur and can be life-threatening, especially when venous return to the heart is significantly diminished. The exact prevalence of CVOs is not well known as they can be incidental imaging findings in asymptomatic patients. , , , This is due to highly effective networks of collateral drainage that reflexively dilate to bypass an in-line blockage and maintain venous return to the heart. CVOs that occur gradually allow for progressive maturation of collaterals, providing equal outflow to obviate venous congestion. Even acute or subacute symptoms from a rapidly progressive CVO can be mitigated by collateral formation overtime, especially with effective anticoagulation to reduce superimposed thrombosis and progression of disease. However, there are many patients in which collateralization and conservative measures are inadequate. This is more common with extensive and progressive CVOs that eliminate available bypass points that prevent collaterals from forming. This not only causes inadequate compensation and notable symptomatic congestion but increases the likelihood of thrombosis and organ dysfunction. A common scenario is when an inciting event (injury, illness, surgery, a period of prolonged immobility) triggers Virchow’s triad, causing acute on chronic progression of a CVO with superimposed thrombosis. This results in more urgent and even emergent presentations.
The core symptoms of venous congestion commonly develop in the upper or lower extremities with swelling (nonpitting edema > pitting), heaviness, pressure-like pain, numbness, tingling, warmth, redness and reduce mobility. Prolonged CVO in the SIC and IIC will eventually cause post-thrombotic syndrome and venous insufficiency from valvular dysfunction and reflux. Data shows a 40%-60% likelihood of inducing moderate to severe PTS (edema, claudication, immobility, ulceration and infection) within 2 years. Clinical scoring using the CEAP classification and Villalta system can help in assessing severity and the benefit of intervention. Patients who exhibit C4 disease (stasis dermatitis) or greater and have moderate or higher Villalta scores generally benefit from intervention both inpatient and outpatient, especially those with nonhealing venous ulcers. Emergent inpatient intervention is usually reserved for venous ischemia with acute phlegmasia. On exam patients can begin with less severe findings of cerulae albens (a milk-white extremity with intact sensorimotor exam), but progress to critical cerulae dolens (purple cyanotic extremity with sensorimotor deficits). CVOs portend a higher risk of phlegmasia than peripheral DVT given the reduction in available collateral pathways. Significant venous pressure more easily builds escalating from vague discomfort and swelling to tense edema with neuropathy, cyanosis and ischemic tissue loss within 6-12 hours from symptom onset. Patients with thrombotic CVO are also at risk for pulmonary emboli (PE) more so in the IIC distribution than the SIC veins. Studies estimate up to 12% of caval thromboses having concomitant PE diagnosed at presentation or on subsequent imaging. Debate exists regarding the comparative risk of PE in the setting of high-risk free-floating clot or clot in-transit, but the literature favors the risk of PE is increased.
Thoracic CVOs of the SIC distribution result in the unique constellation of SVC syndrome, including swelling with facial plethora and visible venous distension in the H&N (increased jugular venous pressure), cough (dry), hoarseness, dysphagia, orthopnea, dyspnea, conjunctival suffusion, vision changes, papilledema, headache, tinnitus, cyanosis, presyncope (lightheadedness and dizziness), syncope, hypotension, altered mental status, stupor and coma. SVC syndrome can be graded from 0-4 (asymptomatic to life-threatening coma and hypotension) using the classification by Yu et al. in 2008 with the most common findings being facial edema. Life-threatening symptoms develop more so when in-line SVC and collateral AzV drainage are both occluded, which critically impairs venous return to the right heart. Inadequate preload and pulmonary perfusion lends to hypotension and hypoxemia while severe congestion causes coma and respiratory distress from cerebral and laryngeal edema. This creates a vicious cycle where intubation and volume resuscitation, though necessary, could cause cardiovascular collapse and brain death. Malignancy accounts for 60%-70% of SVC occlusions, particularly lung cancer, with remaining occlusions being benign device related fibrosis and thrombosis (cardiac leads and central venous catheters). Indwelling cardiac leads regularly form fibrin sheaths and thrombus that promote CVO in the SIC. Patients who develop significant congestion in the ipsilateral extremity can undergo CVR with IVUS. However, interventions are usually limited to venoplasty without stenting. Treatments beyond this require a multidisciplinary approach involving the electrophysiologist and cardiothoracic surgeons for potential laser-assisted lead extraction followed by endovascular reconstruction. Hemodialysis patients are another unique subset that frequently suffer from benign CVO in the SIC veins due to long dwell times of dialysis catheters, continuous high flow from fistulas and grafts and thrombotic complications of accesses. When a CVO is in-line with a dialysis access the symptoms of venous congestion are magnified by the high flow. Moreover, long standing CVOs result in access malfunction with difficult cannulation due to swelling and pain, prolonged bleeding, aneurysmal degeneration, high pressures and recirculation. Prior to any CVR it is critical to know if a patent fistula or graft is present given the much higher risk of morbidity and mortality with rupture or bleeding in an arterialized venous system.
Abdominopelvic CVOs of the IIC distribution also have special features outside of the lower extremities. Swelling and superficial venous dilation can develop circumferentially in the abdominal wall and lower back, especially with IVC involvement. Impaired drainage and occlusion of the gonadal, renal or hepatic veins can cause concomitant pelvic congestion syndrome, renal failure with hematuria and oliguria and Budd-Chiari with liver dysfunction. Pericaval abdominal malignancies, notably renal cell carcinoma (RCC), are a common etiology alongside prolonged IVC filtration and central venous access. Less common etiologies include trauma, surgery, infection, vasculitis, hypercoagulable disorders, pregnancy, renal disease, drugs and dehydration. CVO of the IIC can propagate clot antegrade and retrograde with clot begetting clot exponentially as venous outflow and inflow is progressively reduced. Due to the capacitance of the iliocaval system, a significant burden of thrombus can build asymptomatically until a critical flow-limitation is reached, causing a symptomatic acute on chronic occlusion. At the time of symptomatic detection, a mix of acute (<14 days), subacute (14-28 days) and chronic (> 28 days) thrombus is present in radial layers with softer acute to subacute clot at the central laminar flow and chronic fibrinous clot adhering to the vein wall. Enhancing tumor thrombus should also be suspected when invasive and thrombogenic pericaval cancers, especially RCC, capable of thrombosing the high capacitance iliocaval system are seen on imaging. It is important to be aware of these associations and their pathophysiology to appropriately diagnose and treat CVO of the IIC.
Occlusions of the portal system (PVO) are a unique subset of CVO in the abdomen and pelvis that can involve the mesenteric and splenic veins. Benign etiologies, including cirrhosis, hypercoagulability, pancreatitis, GI infection and inflammation, are more common than malignant occlusions, such as hepatocellular and pancreatic cancer. PVOs can present with abdominal pain, ascites and varices along portosystemic collateral pathways that can bleed. Subacute to chronic mesenteric ischemia can develop causing postprandial pain, anorexia, bleeding, nausea, vomiting and weight loss. However, rarely acute bowel ischemia and infarction do occur and should be suspected in patients with an acute abdomen, sepsis and lactic acidosis. PVO are extremely problematic in cirrhotic patients and often require portal vein recanalization and even creation of a transjugular portosystemic shunt (TIPS) prior to transplantation.
Catheter malfunction and associated thrombosis is an increasingly common presentation warranting CVR in both the SIC and IIC distributions. CVR with IVUS can be used to restore function or open central veins to facilitate placement of a catheter. Occlusions from fibrin sheaths, thrombus and strictures impair aspiration and flushing and cause symptomatic venous congestion. The likelihood of CVO increases with larger French catheters, longer dwell times, greater traversed venous length (PICCs), smaller access vessel, placement across a compression point (TOS) and malposition, especially retracted dialysis catheters that subject the SVC to high flows. , , , ,
Preoperative Imaging
Once clinical concern is present, diagnosis of CVO begins with noninvasive imaging, including a duplex ultrasound of the upper or lower extremity veins (US) and a computed tomographic venogram (CTV). US is often the first test ordered given peripheral DVT symptoms are the most recognizable. Veins can contain acute hypoechoic central clot with venous expansion or chronic echogenic eccentric clot with venous contraction. Specific findings of occlusive (noncompressible) DVT extending centrally into the SIC or IIC veins with loss of respiratory phasicity should trigger a CTV to evaluate the central veins. Adequate contrast filling of the veins on CT requires a minimum of a 2–3-minute delay to distinguish pathology from artifactual mixing of unenhanced venous blood. On CTV acute to subacute clot maintains or expands the venous caliber but generally lacks mature superficial and deep collateral drainage pathways. Conversely, venous stenosis, calcification and large collaterals favor a more chronic occlusion (>28 days), particularly when an obstructing tumor or intravascular device is present. Unfortunately, clot density by Hounsfield units can be variable, but use of dual-energy and spectral CT is being studied for this purpose. However, often mixed chronicity thrombus is present at the time of detection. A CT angiogram of the pulmonary arteries (CTA-PA) and echocardiography should be considered for both emboli and clot in transit, particularly in patients with intracardiac extension of thrombus and hemodynamic sequela of PE or heart failure. Identification of the underlying etiology is critical as well, especially in the setting of malignancy. Contrast enhanced Magnetic Resonance Imaging (MRI) can help differentiate tumor invasion and thrombus from benign CVO. MRI also has a role for reducing radiation dose, particularly in young females, and obtaining noncontrast time of flight imaging in those with contrast allergies and renal dysfunction. Its use, however, is limited by long acquisition times that are hard for patients with symptomatic CVOs to tolerate. , , , , , ,
Additional Preprocedure Evaluation
In addition to the relevant past medical history, review of symptoms and physical examination as detailed above, it is important to confirm if a patient is allergic to contrast, latex, lidocaine, sedatives, heparin/anticoagulants and metals contained in stents and devices (namely Nickel) and adjust accordingly.
Early initiation of anticoagulation using heparin or low-molecular weight heparin and continuation during the procedure is paramount to reducing disease progression and complications from thromboemboli. Active bleeding and other contraindications to anticoagulation and thrombolytics (recent intracranial hemorrhage, surgery, trauma, aortic pathology, coagulopathy and severe thrombocytopenia) must be reviewed as well prior to CVR and could require expert consultation (usually hematology or vascular medicine).
Active infection must also be ruled out, particularly septic thrombophlebitis and cellulitis in affected extremities. Appropriate antibiotics should be initiated prior to intervention and ideally blood cultures should be clear. Of course, scenarios exist where CVR using IVUS is necessary to extract thrombus or occlusive venous device that is a persistent source of infection. The risks and benefits of such a procedure would necessitate the input of an infectious disease specialists for confirmation of the site and medical optimization. In the case of an infected thrombosed hemodialysis access consultation with vascular surgery is warranted given open surgical thrombectomy and debridement are often necessary.
With regards to sedation, most CVR with IVUS are done under general anesthesia given the inherent length and associated discomfort, but also for support in case of complication, such as pulmonary emboli or SVC rupture causing tamponade and shock. An exception to this is May-Thurner interventions, which can be tolerated under moderate sedation. All patients should have an ASA and Mallampati score performed and be assessed for hemodynamic stability, right heart dysfunction, oxygen demand, OSA and ability to lay flat. In the case of right heart strain and SVC syndrome monitored anesthesia care (MAC) without intubation is the safest route for sedation to avoid cardiovascular collapse. However, experts in cardiac anesthesia can perform intubation safely if deemed necessary. preoperative Labs include a CBC (Hgb >7 and Plts > 30 K), CMP (Cr, GFR and LFTs), Blood cultures, Lactate and Coagulation panel (INR, PT, PTT and/or Xa levels) and a pregnancy test. Interventions requiring exposure to x-ray, contrast and sedation in pregnant patients are generally avoided during the sensitive 1st trimester. However, the use of IVUS can mitigate this exposure to the fetus if intervention is necessary. Acute and chronic renal failure should also be assessed before intervention given the possibility of progression with iodinated contrast. Although contrast exposure can be significantly reduced with the use of IVUS, the risks and benefits must be weighed carefully in patients who are not on dialysis. This could require input from nephrology to determine safety and to aid with counseling the patient.
Equipment
In the United States there are 3 companies that provide over the wire radial IVUS catheter systems: the OptiCross catheters from Boston Scientific, the Infraredx Clarispro catheter from Nipro and the Visions PV, Eagle Eye Platinum and Refinity ST catheters from Philips. For CVR it is optimal to use a 0.035” over the wire radial IVUS catheter with a greater field of view (FOV) to encompass the larger central systemic veins and perivenous space. Only 2 radial IVUS catheters, Visions PV .035 (Philips) and OptiCross35 (Boston Scientific), are 0.035” systems. The Visions PV is an 8.5 French compatible 90 cm long catheter with a 64 element cylindrical digital array at the tip generating a 60 mm FOV. The catheter has 1 cm spaced radiopaque markers for 25 cm. It requires a dedicated mobile or integrated computer processing system that can provide continuous live imaging and acquire single images and pullback cine clips. Manual and automated cross-sectional measurements of dimension and doppler flow can be performed on images. Adjustments to the gain, FOV, rotational orientation and any ring down artifact can be made to optimize the image. The OptiCross35 is an 8 French compatible 105 cm long unmarked catheter with a rotating transducer at the tip generating a 70 mm FOV. It requires an analogous dedicated mobile or integrated computer processing system with the same capabilities as above. An exchange length wire (>260 cm) is recommended for both catheters, but the Visions PV could be used over a 180 cm wire if necessary.
Several side-firing Intracardiac Echocardiography (ICE) catheters are available in the United States, including the UltraICE from Boston Scientific, the AcuNav from Siemens, and the ClearICE from St Jude Medical. ICE catheters are steerable catheters ranging from 8-10 French and 90-110 cm in length. They are deployed independently not over a wire via a sheath of corresponding size in the IjV of common femoral vein (CFV). These catheters use a stationary 64-element phased-array mounted along one side of the tip. This provides a wedge-shaped grayscale image from a 90-degree sector with greater depth (14-21 cm) and color and flow doppler capabilities than radial IVUS. The tip can be deflected in the anteroposterior and mediolateral directions to enhance visibility of adjacent regions.
For CVR standard image-guided venous access is obtained using extravascular US, a micropuncture set or 18 G needle and 0.035” guidewire at the appropriate locations under sterile technique. It is not uncommon to need a long micropuncture system for popliteal access in a swollen extremity. Fluoroscopy is used to guide recanalization and intermittently monitor positioning of the IVUS catheter during advancement. Hydrophilic exchange length wires are highly advised during CVR to facilitate traversal of any length occlusion, establish a secure through and through access using a 6 French Goose neck snare and introduce a range of devices without losing wire. The most widely used crossing wires for CVR are the hydrophilic standard and stiff 0.035” Terumo Glidewires, which come with an angled and straight tip. Other 0.035” hydrophilic wires include the Terumo Glidewire Advantage and Cook Medical Roadrunner among others. Crossing with a hydrophilic 0.014” and 0.018” wire systems is also feasible if preferred, but thinner stiff weighted wires are sharper and more capable of perforating on the venous side. Regardless of the selected wire, successful recanalization relies on a stable crossing system to prevent recoil and buckling while traversing a CVO. This requires at least a co-axial system with a sheath and tapered catheter, both of which are braided and hydrophilic ideally. An adequate length and shape should be used to abut the occlusion with the system and steer a wire across it. A 65-135 cm long catheter and a 45-65 cm long sheath is usually sufficient from most access points with shorter 40 cm catheters and 10-25 cm sheaths reserved for IjV approaches to SIC occlusions. This avoids excessive extravascular length of the sheath and catheter. Longer uncontained external components are prone to bending and buckling in free air when an applied forward force transmits up the system. Moreover, longer lengths in certain catheters are inherently flimsier by build and buckle more easily, especially when they extend outside the reinforced lumen of the sheath. This reduces trackability and pushability limiting the advancement and rotation of a catheter through a CVO. The optimal system would also use a tapered catheter married 1:1 with a sheath to maximize the support of the sheath circumferentially around the catheter. Examples of hydrophilic support catheters for crossing CVOs are the Philips QuickCross, Terumo NaviCross, Medtronic TrailBlazer and the Cook Medical CXI with the TriForce system. If a challenging CVO is present, then a telescoping tri-axial system using a 9 French base sheath, a 6 Fr guide sheath or guide catheter at least 10 cm longer than the base sheath and a conventional 4-5 French catheter of at least 100-135 cm in length is best. This allows for telescoping traversal and alternating rotational force to be applied in a calculated and steerable manner. It is important to gradually increase the stiffness of a crossing system and adjust the force and direction of the wire within the lumen to reduce the risk of extraluminal perforation.
Additional equipment and devices that might be necessary include semi-complaint and noncomplaint plain angioplasty balloons ranging from 4-20 cm in diameter and length. A longer length working wire such as a 450 cm Jagwire or Dreamwire from Boston Scientific for taller patients with through and through access form the IjV and popliteal vein. The Inari Medical ProTrieve sheath for embolic protection via the IjV along with a range of devices for pharmacologic and mechanical thrombectomy from Inari (RevCore, VenaCore, ClotTriever and FlowTriever), Penumbra (Indigo System Catheters), Boston Scientific (AngioJet & Ekos), AngioDynamics (AlphaVac System), Argon Medical Devices (Cleaner system), AngioDynamics (Uni-fuse), and Medtronic (Cragg-McNamara).
With regards to stenting CVO, the preferred choice is a bare metal self-expanding stent. However, balloon expandable and self-expanding stent-grafts do play a role in malignant occlusions to exclude tumors and prevent both ingrowth and rupture, after sharp recanalization with an extravascular course, when treating hemodialysis related occlusion to reduce intimal hyperplasia and to exclude venous injuries. Below are commonly used stent systems: Venovo Venous Stent System from BD Interventional, Zilver Vena Venous Self-Expanding Stent from Cook Medical, Abre Venous Self-Expanding Stent System from Medtronic, Wallstent from Boston Scientific, Viabahn & VBX & Aortoiliac Extenders from Gore Medical, and Covera from BD.
Procedural Steps
General Considerations for IVUS
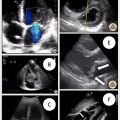
IVUS is a reliable tool to tackle the extensive static and dynamic variations found within normal and pathological veins. As highlighted above, the wide quantitative ranges in the literature preclude extrapolation of sampled venous metrics across patients. Moreover, findings from preprocedural CT and US imaging can evolve by the time an intervention is underway. IVUS allows interventionalists to obtain live cross-sectional ultrasonic images of a CVO to more accurately and precisely treat the individual needs of a patient. Although very helpful for venous mapping, the quality of static or pullback venography at select segments can vary with contrast injection, flow impedance (by sheaths and pathology), variable breathing, cardiac function, motion, radiation exposure and digital calibration. In contrast, IVUS images are only influenced by gain, FOV and infrequent artifacts from adjacent structures (ex. ring down). These are easily overcome using digital adjustments and over the wire centerline positioning. IVUS also provides continuous 360-degree intraluminal imaging across multiple locations and time points. This enables qualitative and quantitative evaluation of a vein’s cross-section throughout the cardiac and respiratory cycle without significantly adding radiation or contrast. ,
Transverse 2-D cross-sectional measurements provide accurate maximum and minimum values of both the luminal and wall to wall area and caliber. Additionally, direct sonographic visualization better discriminates between pathologies, particularly DVT and fibrotic strictures (both of which appear similarly as absent filling on venography), and evaluates vein wall integrity and perivenous tissues to distinguish malignant compression and invasion from benign compression syndromes. Furthermore, it can identify points of rupture with hematoma that have undergone tamponade and are not visible by venography, but still at risk for progression with venoplasty. Continuous dynamic assessments with IVUS also allow for simultaneous evaluation during augmentation of venous volume (infusion of saline), inflow (calf or hand squeeze) and positioning (neutral vs provocative maneuvers). With this range of venous distension and flow it is easier to differentiate transient vasospasm, compression and physiological under distension from true fixed CVOs with slow echogenic flow. Moreover, aggregate measurements of CVOs can be obtained at varying levels of distension to establish an accurate steady state caliber to guide balloon and stent sizing.
General Procedure Steps
Initial selection of the venous access points is critical for successful CVR with IVUS. For nearly all CVOs, and especially those involving the cavae, recanalization is safest when done through dual antegrade and retrograde access. The best vessels to access are in-line patent peripheral veins with the shortest and straightest course to the CVO. This usually includes the Internal Jugular, Brachial, Basilic, Cephalic, Common Femoral, Greater saphenous and Popliteal veins with the right side having more favorable angulation. In many instances the access point also factors in the need for additional recanalization to restore inflow from the periphery to maintain central venous patency. Appropriate selection optimizes geometric alignment for more stable crossing systems capable of pushing and steering through the center of a tough CVO. Co-axial or Tri-axial hydrophilic braided crossing systems (9 Fr base sheath, 6 Fr guide sheath, and 4-5 Fr tapered catheter) can be advanced from both directions until the sheath and catheter reach each end of the occlusion. , At this point heparinization should be considered with the ACT maintained between 230-250 seconds.
Both blunt and sharp recanalization can then be attempted from above or below with a target in the opposing patent end to guide traversal in the true lumen. Venography and over the wire radial IVUS should be used to identify a concavity or nub at the cranial or caudal margin of the occlusion. If present the sheath can be pointed directly at this point to support seeding the tip of a 4-5 French catheter into the nub. A standard or stiff Glidewire should then be driven a short length into occlusion without dislodging the support system. Fluoroscopically it will appear as if the wire exited the catheter and immediately stuck into a tight space. Resistance with movement of the wire or catheter suggests appropriate seeding within the occlusion. The wire can then be spun using a rotary motion to slowly drill through the occlusion. A similar degree of resistance should remain until the wire reaches the target at the opposing patent end. If the wire stops advancing and begins buckling back, then carefully track the catheter as far along the wire as possible without causing it to retract. To aid in trackability the catheter can be gently spun over the wire to help bore through. A sudden loss of resistance or an atypical wire course prior to reaching the patent end raises concern for extravascular perforation, especially if no collateral is present in the region. A safe and easy way to confirm if the wire has reached the target is by placing IVUS in the opposing true lumen. If it is not seen sonographically and oblique fluoroscopic projections show malalignment, then the wire should be retracted to the point just before the resistance was lost and then redirected back into a point of resistance prior to drilling forward. Several tries using blunt recanalization with an array of wires and crossing systems should be attempted prior to transitioning to a sharp technique. , ,
Sharp techniques are best suited for short relatively straight native CVO, but longer in-stent occlusions can be safely traversed with a sharp approach. The tools available for sharp recanalization in escalating order are the Back end of a stiff 0.014-0.035” wire, Needle cannulas/sheaths (Colapinto 16 G, Rosch-Uchida 14 G, Chiba, Trans-septal) and PowerWire Pro radiofrequency guidewire. Both radial and side-firing IVUS can be used for targeting and direct visualization of entry in the opposing lumen. Adjacent side-firing catheters have the added ability to monitor a sharp trajectory in real-time. By deflecting the tip, the sector can be positioned to view across the entirety of the recanalization length with all 3 available tools for sharp approaches appearing echogenic. It is also feasible to place an easy to reach target, such as a balloon, snare or Amplatzer plug next to the IVUS catheter.
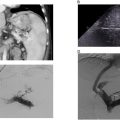
Once wire is visibly across to the other end on IVUS a snare can be used to establish stable through and through access. The wire can then be exchanged for a rail or working wire, such as 0.035” exchange length 260 cm Ampltaz or 450 cm Dreamwire. IVUS is then advanced across the unopacified occlusion to confirm the true lumen was traversed and evaluate the underlying pathology. Predilation can be done with a 4 mm balloon to improve trackability of the IVUS catheter through the CVO if necessary. Visualization of the catheter within intact vein walls and identification of normal adjacent vessel segments and perivenous structures helps to confirm recanalization through the true lumen ( Fig. 1 ). For example, seeing a pulsatile iliac artery parallel to the target iliac vein supports an in-line intraluminal course.