Brain and Its Coverings: Introduction
Technological advances in radiology during the past 30 years have vastly improved our ability to diagnose neurologic diseases. Prior to the introduction of computed tomography (CT) in 1974, neuroradiologic examinations of the brain consisted primarily of plain films of the skull, cerebral arteriography, pneumoencephalography, and conventional nuclear medicine studies. Unfortunately, these techniques, for the most part, provided only indirect information about suspected intracranial processes, were insensitive in detecting subtle or early brain lesions, or were potentially harmful to the patient. Computed tomography revolutionized the radiologic workup of central nervous system (CNS) abnormalities because for the first time normal and abnormal structures could be directly visualized with minimal risk to the patient.
In the late 1980s, it became apparent that magnetic resonance (MR) imaging would become the procedure of choice for evaluating many neurologic disorders, as well as for demonstrating vascular flow phenomena. Since then, there have been many technological advances associated with this modality. These include improvements in magnet and coil design, decrease in imaging time, and the development of new pulse sequences. In addition to advances in conventional anatomic imaging, there has also been substantial growth of “physiologic” MR imaging including MR spectroscopy (MRS), diffusion-weighted (DW) and perfusion-weighted (PW) MR imaging, and functional MR imaging (fMRI), among others. These imaging modalities provide functional information about the brain and have the potential to greatly extend our understanding of neuropathology beyond structure alone.
Revolutionary breakthroughs in CT scanning technology during the 1990s facilitated the development of advanced CT applications, namely, dynamic contrast-enhanced CT angiography (CTA) and CT perfusion (CTP). These techniques, which allow high spatial resolution imaging of the cervical and intracranial vasculature, are currently being used in the evaluation of the acute stroke patient in many medical centers. Furthermore, recent technologic advances in CT imaging have markedly decreased scan times and have allowed evaluation of very tiny anatomic structures because of improvement in spatial resolution.
Recent advances in nuclear medicine functional imaging techniques, including single photon emission computed tomography (SPECT) and positron emission tomography (PET), improvements in conventional angiographic methods, and expansion of catheter-based therapeutic procedures have provided the neuroradiologist today with an even greater variety of strategies for diagnosing and treating neurologic abnormalities.
The main purpose of this chapter is to acquaint the reader with the major radiologic techniques used currently to evaluate the brain and its coverings. The strengths and weaknesses of these techniques are discussed. Imaging anatomy of the brain and its coverings is briefly reviewed. Basic guidelines pertaining to technique selection for evaluating common neurologic conditions are provided. Finally, examples of common brain abnormalities are presented. It is assumed that readers have a basic understanding of neuroanatomy and neuropathology.
Although this chapter may give some insight into neuroradiologic study interpretation, that is not its primary goal. Rather, readers should expect to become reasonably familiar with the various techniques employed to examine the brain and should gain some idea about the appropriate ordering of examinations in specific clinical situations.
Techniques
Radiologic modalities useful in evaluating the brain and its coverings can be divided into two major groups: anatomic modalities and functional modalities. Anatomic modalities, which provide information mostly of a structural nature, include plain films of the skull, CT, MR imaging, cerebral arteriography (CA), and ultrasonography (US). On the other hand, SPECT and PET imaging, CT perfusion, DW and PW MR imaging, fMRI, and MRS are primarily functional modalities, which give information about brain perfusion or metabolism. Some techniques provide both anatomic and functional information. For example, cerebral arteriography depicts blood vessels supplying the brain but also allows us to estimate brain circulation time. Ultrasound of the carotid bifurcation is another modality that provides both anatomic and functional information. A routine sonogram of the carotid bifurcation gives anatomic data that, when combined with Doppler data, readily provides information about blood flow.
The following discussion of current neuroradiologic techniques emphasizes relative examination cost and patient risk, along with the advantages and disadvantages of each technique. The normal imaging appearance of the brain and its coverings is also illustrated.
Plain radiographs of the skull are obtained by placing a patient’s head between an x-ray source and a recording device (ie, x-ray film). Whereas bones of the skull attenuate a large number of x-rays to create an image, soft tissues such as scalp or brain are poorly visualized, if at all. Another difficulty in plain film interpretation results from the spherical shape of the skull, leading to multiple superimposed structures. The resultant skull radiograph primarily gives information about the bones of the skull, but no direct information about the intracranial contents. Indirect information about intracranial abnormalities can sometimes be obtained from the skull plain radiograph, although this information can be quite subtle, even in the setting of advanced disease. Skull plain radiographs have been largely replaced today by more sensitive techniques such as CT or MR imaging. Even in the setting of suspected skull fracture, plain radiographs are rarely indicated, because CT scans also show the fracture, as well as any intracranial abnormality that might require treatment. Currently, plain radiographs of the skull serve a very limited role in routine neuroimaging and are only briefly discussed.
CT scans consist of computer-generated cross-sectional images obtained from a rotating x-ray beam and detector system. Advances in scanning technology now permit simultaneous acquisition of multiple images during a single rotation of the x-ray tube (eg, currently up to 256 slices) during a breath-hold. The resultant images, unlike plain films, exquisitely depict and differentiate between soft tissues, thus allowing direct visualization of intracranial contents and abnormalities associated with neurologic diseases. The contrast or brightness (“window” or “level,” respectively) of these images can be adjusted to highlight particular tissues.
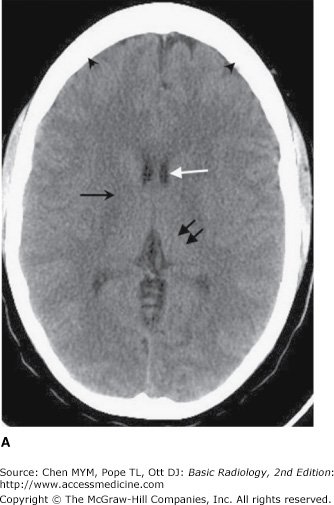
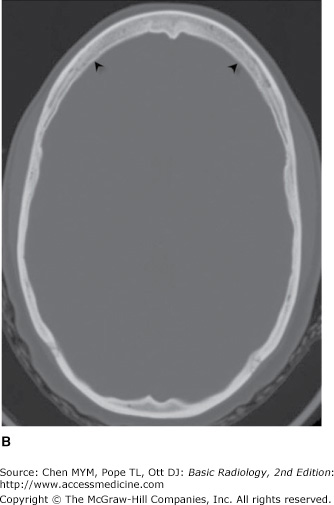
Typically, a head CT consists of images adjusted to emphasize soft-tissue detail (soft-tissue windows) as well as images adjusted to visualize bony detail (bone windows) (Figure 12-1). As stated earlier, CT image generation is dependent on variable attenuation of the x-ray beam based on the density of structures it passes through (eg, bones of the skull base are very dense and attenuate a large percentage of the x-ray beam). Therefore, cortical bone appears white (has a high attenuation value or Hounsfield unit), whereas air within the paranasal sinuses appears black (has a low attenuation value) (Figure 12-1). Cerebral white matter has a slightly lower Hounsfield number than does cerebral gray matter and consequently appears slightly darker than gray matter on a head CT scan (Figure 12-1 A). Intracranial pathologic conditions can be either dark (low attenuation) or bright (high attenuation), depending on the particular abnormality. For example, acute intracranial hemorrhage is typically very bright, whereas an acute cerebral infarction demonstrates low attenuation when compared to the surrounding normal brain because of the presence of edema.
Figure 12-1.
Normal axial head CT images. Appropriate window selection allows visualization of both intracranial contents (A) and bony calvarium (B). Note differences in attenuation among gray matter (left thalamus, double black arrows), right internal capsule (single black arrow), cerebrospinal fluid (CSF; frontal horn of the left lateral ventricle, white arrow), and bone (skull, arrowheads).
The CT technologist can change the slice thickness and angulation, among other technical factors, to alter the way an image appears. Images are typically obtained axially in helical fashion, with acquisition of a volumetric data set. Current scanner technology allows the axial data set to be reformatted in coronal, sagittal, or oblique planes or as a 3-D image, with little, if any, loss of resolution. CT examinations may be performed after intravenous administration of an iodinated contrast agent, especially when MRI is contraindicated or unavailable. These agents “light up” or enhance normal blood vessels and dural sinuses, as well as intracranial structures that lack a blood-brain barrier (BBB), such as the pituitary gland, choroid plexus, or pineal gland. Pathologic conditions that interrupt the BBB (such as neoplasm, infection, or cerebral infarction) also demonstrate enhancement after contrast material administration. For this reason, lesions that may be invisible on a noncontrast study are often obvious on the contrast-enhanced scan.
The intravenous administration of a contrast bolus can be appropriately timed to maximize vascular opacification of the arterial or venous circulation (CTA or CTV, respectively). These high spatial resolution 3-D CTA images (Figure 12-2) of the cervical and intracranial vasculature are routinely employed to quantify vessel stenosis due to atherosclerotic disease, to assess for vascular injury related to trauma, or to detect cerebral aneurysm in the patient with subarachnoid hemorrhage.
In particular, CTA has become a standard component of evaluating the acute stroke patient. CTA accurately identifies the location and extent of large vessel occlusions and can be supplemented by a more detailed, quantitative evaluation of the cerebral microvascular hemodynamics (CT perfusion) during the early phase of bolus passage. Software analysis of this tailored CTA data produces maps of capillary-level cerebral perfusion, typically measured by mean transit time (MTT), cerebral blood flow (CBF), and cerebral blood volume (CBV). In the setting of cerebral infarction, these parameters can help interpret the infarct “core” (CBV) versus the ischemic “penumbra” (MTT and CBF). Evaluation of potential mismatch between the infarct core and surrounding penumbra serves as the rationale for instituting various reperfusion techniques.
Another recent application of CTA is in the screening evaluation of blunt cerebrovascular injury, including closed head injuries, seatbelt abrasion (or other soft-tissue injury) of the anterior neck, basilar skull fracture extending through the carotid canal, and cervical vertebral body fracture. It is an accurate technique for detecting internal carotid artery (ICA) dissections and for assessing stenoses, although evaluation is difficult in areas of surrounding dense bone as a result of associated “streak artifact.” However, this noninvasive, relatively short imaging procedure rivals conventional angiographic methods, as it requires no patient transfer and can sensitively identify vascular injury in relation to other associated brain insults, cervical spine injury, or facial or basilar skull fractures.
High-resolution data acquisition during the venous phase following intravenous contrast administration (CT venography) can be used to identify dural sinuses and cerebral veins, evaluate for dural venous sinus thrombosis, and distinguish partial sinus obstruction from venous occlusion in the setting of adjacent brain masses. CT venography can also differentiate slow flow from thrombosis, which may occasionally be difficult with MR techniques.
The major advantages of CT are that it is inexpensive, is widely available, can be used in patients with MR-incompatible hardware, and allows a relatively quick assessment of intracranial contents in the setting of a neurological deficit. The images obtained are very sensitive to the presence of acute hemorrhage and calcification, and images revealing exquisite bony detail of the skull and skull base can be acquired. Because of the configuration of the scanner, patients are reasonably accessible for monitoring during the examination.
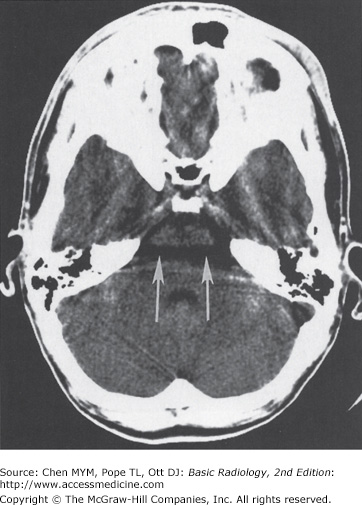
CT scanners do have a number of disadvantages, however. Patients are exposed to ionizing radiation and iodine-based contrast agents (although lower doses of contrast are needed with newer multidetector scanners). Imaging artifacts can interfere with accurate interpretation. In particular, images of the brainstem and posterior fossa are often degraded by “streak artifacts” from dense bone (Figure 12-3). Streak artifacts from metallic objects (eg, fillings, braces, surgical clips) can also obscure abnormalities. Images can be severely degraded by patient motion. Fortunately, unlike MR scans, individual CT images degraded by motion can be rapidly reacquired.
One of the most exciting developments in radiology during the past 30 years has been the growth of magnetic resonance imaging (MRI), which is currently the mainstay of clinical neuroimaging. The concept of nuclear magnetic resonance (NMR), initially used for probing the physiochemical structure of molecules, was first described in the 1930s, but it took more than 40 years before the translation of NMR phenomena could be used for clinical imaging.
MR examinations, like CT scans, consist of computer-reconstructed cross-sectional images (Figure 12-4). In MR imaging, however, unlike CT scans or plain radiographs, the information collected is not x-ray beam attenuation. The MR image is a visual display of NMR data collected principally from nuclei within body tissues—especially hydrogen nuclei within water and fat molecules. Intrinsic tissue relaxation occurs by two major pathways, called longitudinal, or T1, and transverse, or T2, decay. MR imaging sequences that emphasize T1 decay are commonly referred to as T1-weighted; sequences that accentuate T2 relaxation properties are called T2-weighted (Figure 12-4). Most MR scans of the brain use both of these sequences, because certain abnormalities may only be obvious on one or the other. T2-weighted images are usually easy to identify because fluid (eg, cerebrospinal, globe vitreous) is very bright; fluid on a T1-weighted scan is usually dark. Fat is bright on T1-weighted scans, but darker on T2-weighted images. On the other hand, both cortical bone and air are very dark on all imaging sequences. Brain tissue has intermediate intensity; vessels can have almost any signal, depending on the velocity of flowing blood.
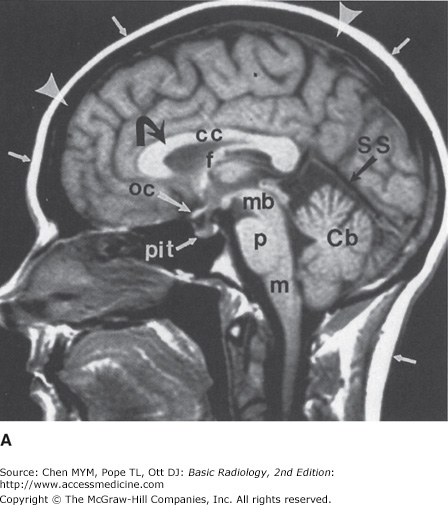
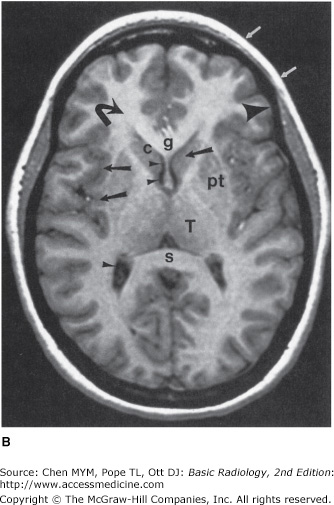
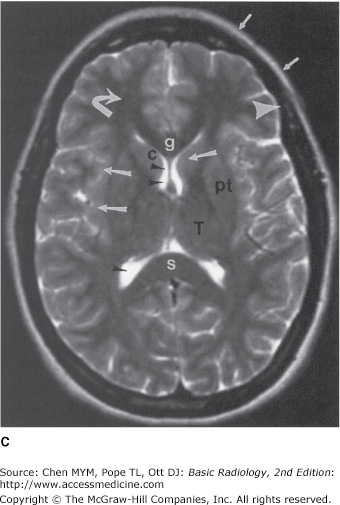
Figure 12-4.
Normal head MR images. Sagittal T1-weighted (A), axial T1-weighted (B), and axial T2-weighted (C) images. Note differences in signal between gray matter (large arrows), white matter (curved arrows), CSF (small arrowheads), fat (small arrows), and cortical bone (large arrowheads) on different pulse sequences. Normal structures include the genu (g) and spleniurn (s) of the corpus callosum (cc), fornix (f), optic chiasm (oc), pituitary gland (pit), midbrain (mb), pons (p), medulla (m), cerebellar vermis (Cb), straight sinus (SS), caudate head (c), putamen (pt), and thalamus (T).
The most commonly used clinically approved contrast agent for MR imaging is gadopentetate-dimeglumine or Gd-DTPA, which is very well tolerated and generally safe, although caution must be used in patients with renal impairment because of the associated risk of developing nephrogenic systemic sclerosis (refer to Chapter 1). Its major use in the CNS is to improve lesion detectability by “lighting up” pathologic conditions that either lack a BBB or have a disrupted BBB.
Conventional MR imaging depicts excellent soft-tissue contrast. Traditionally, long image acquisition times, image artifacts related to patient motion, and the increased cost of scanning due to limited patient throughput have hampered the clinical utility of MR imaging. Over the past 15 years, technical advances in gradient technology, coil design, image reconstruction algorithms, contrast administration protocols, and data acquisition strategies have accelerated the development and implementation of fast imaging methods. These techniques, including fast gradient echo imaging, fast spin echo imaging, FLAIR (fluid-attenuated inversion recovery), and echo planar imaging, have enabled substantial reductions in imaging time. Images may be acquired during a single breath-hold on a clinical scanner, eliminating respiratory and motion artifacts. Vessel conspicuity can be enhanced by application of fat-suppression sequences, which eliminate unwanted signal from background tissues. These improvements have led to a vast range of applications that were previously impractical, including high-resolution MRA, DW and PW MR imaging, MRS, fMRI, and real-time monitoring of interventional procedures.
Since its first clinical application nearly 15 years ago, MRA has proven to be a useful tool for evaluation of the cervical or intracranial carotid vasculature. MRA represents a class of techniques that utilize the MR scanner to noninvasively generate three-dimensional images of the carotid or vertebral-basilar circulations. Although a detailed discussion of these techniques is beyond the scope of this chapter, several comments are noteworthy. These methods permit distinction between blood flow and adjacent soft tissue, with or without administration of intravenous contrast. As noted earlier, revolutionary developments have permitted MRA images to be rapidly acquired with ever-improving temporal and spatial resolution.
Presently, MRA serves as one of the first-line studies for evaluation of arterial occlusive disease and for screening of intracranial aneurysms. These methods have largely replaced conventional arteriographic studies for evaluation of atherosclerotic disease, except in cases of critical stenosis (>70%). In these instances, the degree of luminal narrowing may be overestimated by MRA and may require verification with CTA, catheter-based study, or Doppler ultrasound. Moreover, aneurysms detected on an intracranial MRA typically require a catheter-based study for detailing aneurysm size and orientation, for establishing the location of adjacent vessels and collateral flow, and for confirming suspicious vascular dilatation, as well as for detecting the presence of vasospasm or additional aneurysms that may not be readily apparent on the MRA study. In an increasing number of cases, catheter-based studies will additionally be performed for coil embolization (obliteration) of detected aneurysms, rather than surgical clipping.
Molecular diffusion, the random translational movement of water and other small molecules in tissue, is thermally driven and is referred to as Brownian motion. Over a given time period, these random motions, expressed as molecular displacements, can be detected using specifically designed diffusion-sensitive MR sequences. A common application of diffusion imaging is the detection of early ischemic infarction, where the infarcted tissue “lights up” because of a “restricted diffusion” state within the intracellular compartment. Other applications of diffusion-sensitive sequences include differentiating cysts from solid tumors, as well as evaluating inflammatory/infectious conditions (encephalitis, abscess) or white matter abnormalities (hypertensive encephalopathy).
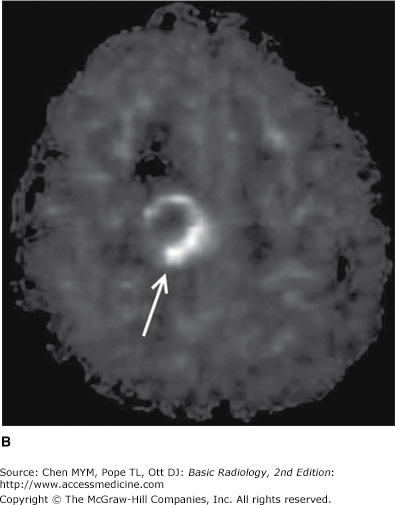
Perfusion MR imaging measures cerebral blood flow at the capillary level of an organ or tissue region. Perfusion-weighted MR imaging has applications in the evaluation of a number of disease states, including cerebral ischemia and reperfusion, brain tumors (Figure 12-5), epilepsy, and blood flow deficits in Alzheimer’s disease. In addition, the close spatial coupling between brain activity and CBF permits the application of perfusion MR techniques to imaging brain function. MR perfusion imaging is technically complex and requires advanced scanner and postprocessing software for image generation. Various methods can be employed including contrast bolus technique (analogous to CT perfusion) or arterial spin labeling (ASL). ASL uses a radiofrequency pulse to “label” protons flowing in the cervical arteries and that signal is subsequently imaged as those protons flow into the cerebrum. One of the major advantages of ASL is that it requires no contrast administration, which is of great benefit in patients with renal impairment.
Figure 12-5.
MR images of a newly diagnosed high-grade glioma. (A) Axial postcontrast T1 image shows a peripherally enhancing, centrally necrotic mass in the right frontal lobe (black arrow) as well as surrounding hypointense T1 signal consistent with vasogenic edema (white arrow). (B) Cerebral blood flow image (pulsed arterial spin labeling technique) shows increased perfusion (arrow) along the peripheral aspect of the mass.
Functional MR imaging is an important brain mapping technique that uses fast imaging techniques to depict regional cortical blood flow changes in space and time during performance of a particular task (eg, flexion of the index finger). The utilization of this technique to localize brain activity is historically based on measurable increases in cerebral blood flow (and blood volume) with increased neural activity, referred to as neurovascular coupling. The hemodynamic response to a stimulus is not instantaneous, but on the order of a few seconds. Consequently, fMRI techniques are considered an indirect approach to imaging brain function, but provide excellent spatial resolution and can be precisely matched with anatomic structures. Changes in blood oxygenation and perfusion can be imaged using fMRI techniques, which has become the most widely used modality for depicting regional brain activation in response to sensorimotor or cognitive tasks.
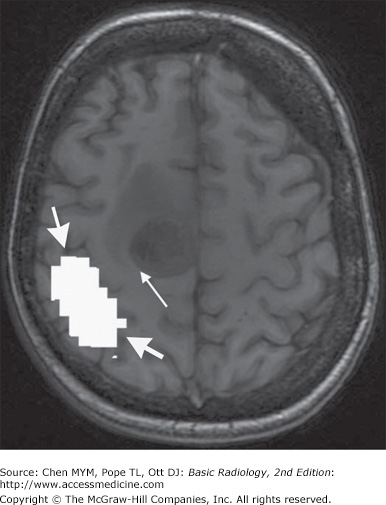
An important clinical application of fMRI is presurgical mapping, whereby eloquent brain cortex can be defined in relation to mass lesions (Figure 12-6). This allows for the judicious selection of an appropriate management strategy (surgical versus nonsurgical) according to the functional nature of the adjacent brain tissue. A second application involves determination of the cerebral hemisphere responsible for language and memory tasks in a patient with complex partial seizures, prior to undergoing temporal lobectomy. Additionally, several groups have reported successful functional activation studies for lateralizing language preoperatively utilizing fMRI.
MR spectroscopy (MRS) provides qualitative and quantitative information about brain metabolism and tissue composition. This functional analysis is based on detecting variations in the precession frequencies of spinning protons in a magnetic field. One factor influencing the precession or resonance frequency is the chemical environment of the individual proton. Protons in different cerebral metabolites can be sensitively discriminated on this basis, and the position of these metabolites can be displayed as a spectrum. The x-axis position of a given metabolite reflects the degree of “chemical shift” of the metabolite with respect to a designated reference metabolite and is expressed in units of parts per million (or ppm). The area under the peak is determined by the number of protons that contribute to the MR signal.
The major metabolites detected in the CNS are N-acetyl aspartate (NAA), a neuronal marker; choline, a marker for cellularity and cell membrane turnover; creatine, a marker for energy metabolism; and lactate, a marker for anaerobic metabolism. In addition to these metabolites, others have been assessed, including alanine, glutamine, myoinositol, and succinate, using various MR strategies. Presently, MRS is being used in clinical practice to provide functional information regarding many CNS abnormalities, and complements the conventional MR imaging study. A common application relates to the pre- and posttreatment evaluation of brain tumors, with MRS playing an important role in assessing for residual or recurrent tumor following surgical resection.
MR imaging offers a number of advantages over CT in the workup of patients with neurologic disease. Its soft-tissue contrast resolution is superior to that of CT, and lesions that may be subtle or invisible on CT are frequently obvious on MR imaging. MR imaging also allows acquisition of multiplanar views in the sagittal, axial, coronal, and oblique projections that may be impossible to obtain with CT. Furthermore, MR imaging gives information about blood flow without the need for a contrast agent, and bony streak artifacts that obscure lesions of the brainstem and cerebellum on CT scans are not present on MR images. Finally, MR imaging does not expose the patient to ionizing radiation.
Cerebral arteriography involves the injection of water-soluble contrast material into a carotid or vertebral artery. Contrast material is injected into the desired vessel via a small catheter, which has been introduced into the body through the femoral or brachial artery. Information about the arterial, capillary, or venous circulation of the brain is recorded on serial plain films or, most commonly, digitized for viewing on a monitor or for storage within a computer (Figure 12-7).
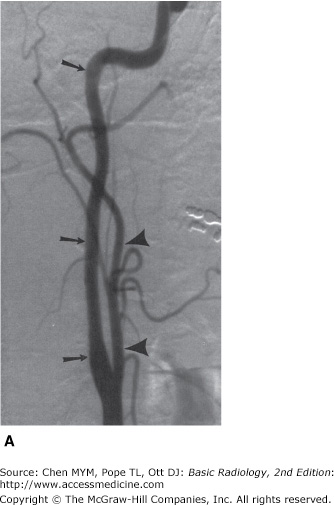
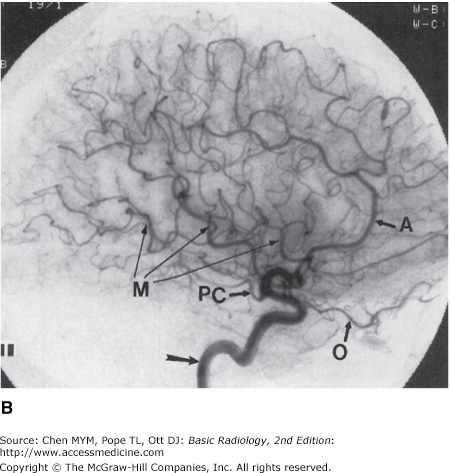
Figure 12-7.
Normal cerebral arteriogram. (A) Lateral view of the cervical carotid artery. Catheter is located within the common carotid artery, and contrast material fills internal (arrows) and external (arrowheads) carotid arteries. (B) Lateral view of the head after injection of the carotid artery (arrow). Note anterior cerebral (A), ophthalmic (O), posterior communicating (PC), and middle cerebral (M) branches.
Cerebral arteriograms are expensive (two to three times as much as MR examinations) and are relatively more risky procedures than other noninvasive neuroradiologic studies. The major risk of the procedure is stroke, which may occur in one of every 1,000 patients. Stroke during cerebral arteriography occurs either from an embolic event (eg, inadvertent injection of air, thrombus formation on the catheter tip, atherosclerotic plaque dislodged by catheter manipulation) or from catheter-related local vessel trauma (eg, dissections or occlusions).
Although CT angiography has largely replaced catheter angiography for most routine diagnostic evaluations, catheter angiography is invaluable in the workup of vascular diseases affecting the CNS. Specifically, it remains the gold standard for assessing vasculitis and is indispensable in evaluating and treating cerebral aneurysms and certain intracranial vascular malformations or fistulas. It is a useful adjunct to cross-sectional imaging (CTA, MRA, or US) to assess vascular stenosis as well as carotid or vertebral artery integrity after trauma to the neck, especially in the setting of acute neurological deficit. Finally, it is unsurpassed for showing vascular anatomy of the brain and is, therefore, useful as a preoperative road map.
The field of interventional neuroradiology continues to grow and exert considerable impact on the diagnosis and treatment of certain CNS diseases. New catheter designs and materials, recently developed endovascular devices (extracranial/intracranial stents), and an increasing number of trained specialists performing endovascular procedures have led to novel therapeutic applications and approaches for managing previously untreatable conditions. Endovascular diagnostic and therapeutic procedures, based on fundamental cerebral arteriography principles, have gained widespread acceptance and, in some cases, rival traditional neurosurgical approaches in terms of complication rates, clinical outcomes, and long-term survival benefit. Although a full discussion of these techniques is beyond the scope of this chapter, they include pharmacologic and mechanical thrombolysis of intracranial clot in the setting of acute infarction or dural sinus thrombosis; embolization (obliteration) of intracranial aneurysms using thrombosing material (ie, coils); carotid artery angioplasty and/or stent placement for critical stenotic narrowing or radiation-induced arterial stricture; preoperative or definitive devascularization of a hypervascular mass or arteriovenous malformation; embolization of small, bleeding external carotid artery branches in epistaxis; balloon occlusion tests of the carotid artery; and endovascular treatment of vasospasm. Embolization materials include particulate emboli, liquid adhesive glues, and various coils.
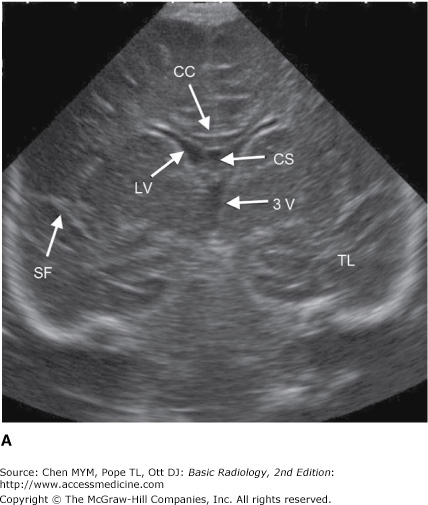
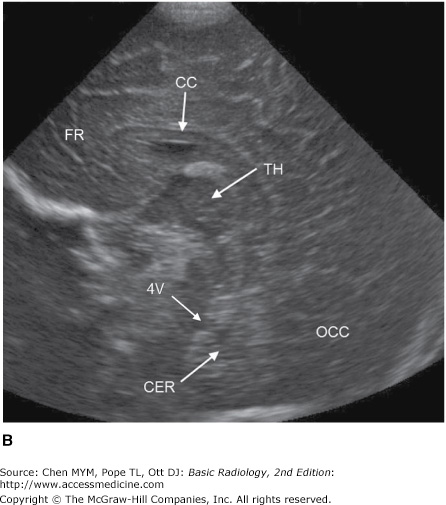
Ultrasonography is the diagnostic application of ultrasound to the human body. Major applications of ultrasonography in CNS disease include gray-scale imaging and Doppler evaluation of carotid artery patency and flow in the setting of atherosclerosis, assessment of vasospasm in the setting of subarachnoid hemorrhage using transcranial Doppler, screening evaluation of intracranial abnormalities in the newborn and young infant (Figure 12-8), and detection of intracranial hemorrhage in premature infants prior to extracorporeal membrane oxygenation therapy. Ultrasound has also been used intraoperatively to demonstrate the spinal cord and surrounding structures during spine surgery and to define tumor and cyst margins during craniotomies.
Figure 12-8.
Coronal (A) and sagittal (B) head ultrasound of a neonate. Normal structures include the corpus callosum (CC), lateral ventricle (LV), cavum septum pellucidum (CS), sylvian fissure (SF), third ventricle (3V), fourth ventricle (4V), temporal lobe (TL), frontal lobe (FR), occipital lobe (OCC), cerebellum (CER), and thalamus (TH).
Transcranial Doppler is a recently developed tool in the evaluation of cerebrovascular disorders. It uses low-frequency sound waves to adequately penetrate the skull and produces spectral waveforms of the major intracranial vessels for evaluation of flow velocity, direction, amplitude, and pulsatility. Present clinical applications include diagnosis of cerebral vasospasm, evaluation of stroke and transient ischemic attack, detection of intracranial emboli, serial monitoring of vasculitis in children with sickle cell disease, and assessment of intracranial pressure and cerebral blood flow changes in patients with head injury or mass lesions.
Ultrasound examinations, although moderately expensive, are virtually risk-free to the patient, involve no ionizing radiation, and are portable (ie, can be performed at the bedside). However, examination quality and therefore diagnostic accuracy are operator-dependent. Also, the heavy reliance of ultrasonography on the presence of an adequate “acoustic window” through which an examination can be performed diminishes its usefulness in examining the brain after the fontanelles close in infancy. Finally, to the untrained eye, anatomic structures and pathologic processes as depicted by US are not as readily apparent as they are on CT or MR images.
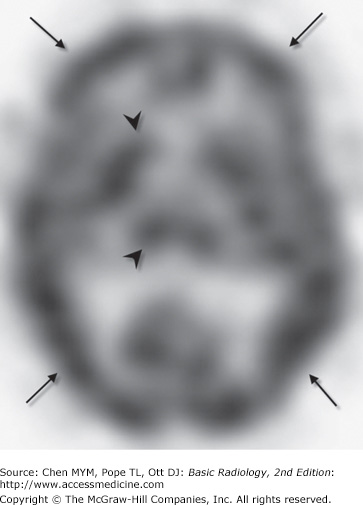
SPECT uses a rotating gamma camera to reconstruct cross-sectional images of the distribution of a radioactive pharmaceutical that has been administered to a patient (usually intravenously). For brain imaging, radioactive iodine (123I) or technetium (99mTc) is combined with a compound that rapidly crosses the BBB and localizes within brain tissue in proportion to regional blood flow. The rotating gamma camera detects gamma rays emitted by the radiopharmaceutical and produces cross-sectional images of the brain that are really a map of brain perfusion (Figure 12-9). SPECT imaging also gives indirect information about brain metabolism, because perfusion is usually highest to parts of the brain with high metabolic activity and lowest to areas with low metabolic demand. Normal SPECT examinations demonstrate activity concentrated primarily in areas of high perfusion/metabolism, such as the cortical and deep gray matter (Figure 12-9).
SPECT studies are moderately expensive (as much as or more than brain MR imaging), and, as expected, they provide limited anatomic information. SPECT also exposes patients to ionizing radiation. Because patients rarely have allergic reactions to the radiopharmaceuticals used, the examination is of low risk. Although SPECT provides critical information regarding regional cerebral perfusion, particularly in the setting of stroke, this information can be more readily obtained during CTA/CT perfusion or MR perfusion acquisitions. SPECT has also been used with varying degrees of success in the workup of patients with epilepsy or dementia.
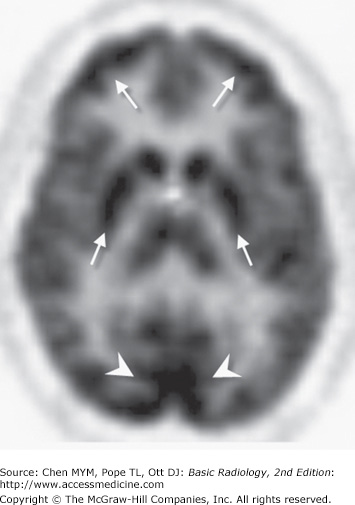
PET scans consist of computer-generated cross-sectional images of the distribution and local concentration of a radiopharmaceutical. This technique is very similar to SPECT imaging; however, there are differences in the type of camera and radiopharmaceuticals used. PET studies use radiopharmaceuticals labeled with a cyclotron-produced positron emitter, which are very expensive to produce and have a very short half-life (on the order of seconds to minutes). The most widely used radiotracer is 18F-deoxyglucose. PET scanning with this agent gives a measurement of brain glucose metabolism. Areas of high metabolic activity (ie, cerebral cortex, deep gray nuclei) demonstrate greater radiopharmaceutical uptake than do areas of low metabolic activity, such as white matter or cerebrospinal fluid (Figure 12-10). The bones of the skull and scalp soft tissues are, for the most part, invisible. Other agents are useful in assessing regional cerebral blood flow, neuroreceptor function, and the like.
Figure 12-10.
Normal axial image of brain PET scan. As in the SPECT study (Figure 12-10), areas of high activity correspond to metabolically active gray matter (arrows), especially the visual cortex (arrowheads).
Since the previous edition, PET scans have become much more widely available, although they remain expensive. The expense, in large part, is related to the cost of imaging equipment and in the production or delivery of radiopharmaceuticals. Although patients undergoing PET examinations are exposed to ionizing radiation, the overall risk to the patient is low. Anatomic resolution, although not as good as with CT or MR imaging, is better than with SPECT imaging. The major advantage of PET imaging is that it is extremely versatile, providing in vivo information about brain perfusion, glucose metabolism, receptor density and, ultimately, brain function.
PET provides useful information in the setting of stroke, epilepsy, dementia, and tumors. At present, the two main indications are in the workup of patients with complex partial seizures and in identifying tumor recurrence in patients who have undergone surgery, radiation therapy, or both, for brain tumors.
Technique Selection
The primary goal of a radiologic examination is to provide useful information for disease management. Radiologic studies can provide a diagnosis or can give information about disease extent or response to treatment. In the present medical climate, it has also become imperative that radiologic workups be performed efficiently and in a cost-effective manner. This requirement presents a problem for clinicians trying to decide which test to order in a given clinical situation.
The major strengths and weaknesses of neuroradiologic examinations have been discussed earlier in this chapter. The following brief discussion concerns the appropriate ordering of examinations in clinical situations. Several points should be emphasized. First, although a recommended modality may clearly be superior to another in evaluating a particular neurologic condition, the choice of examination is not always obvious before the diagnosis is established. For example, in patients with nonfocal headache, MR scans are more sensitive than CT scans for detecting most intracranial abnormalities. However, if the headache is produced by subarachnoid hemorrhage, CT would be a much better examination than MR imaging, because subarachnoid hemorrhage is nearly invisible on MR images. Choice of examinations may also be limited by what is locally available. If MR imaging is unavailable, or if the MR scanner is of poor quality or the interpreting radiologist is inadequately trained in MR image interpretation, then CT would be an excellent examination for evaluating most neurologic disorders.
Next, it is important to realize that the least expensive examination is not always the best first choice, even in this cost-conscious age. For example, most suspected skull fractures should be evaluated with CT scanning and not with plain films, despite the significant cost differential, because what is really important in management decisions is not the fracture itself but the potential underlying brain injury. Some neurologic diseases require multiple radiologic studies for accurate evaluation. Complex partial seizures refractory to medical management frequently require multiple examinations to localize the seizure focus prior to temporal lobectomy. Such a workup normally includes MR imaging and ictal/interictal SPECT and/or PET scanning of the brain, as well as a cerebral arteriogram to identify cerebral dominance.
Finally, certain examinations are contraindicated in certain patients, and an alternative test must suffice. Patients with ferromagnetic cerebral aneurysm clips or pacemakers should not undergo MR imaging. Patients with a strong history of allergic reaction to iodinated contrast media should not routinely undergo contrast-enhanced CT scanning, unless they are pretreated with anti-inflammatory agents (ie, steroids). MR scanning is frequently unsuccessful in claustrophobic or uncooperative patients unless they are sedated.
Congenital anomalies of the brain are best evaluated by MR imaging. MR imaging is the best examination for demonstrating intracranial anatomy. It provides excellent discrimination between gray matter and white matter, superb views of the posterior fossa and craniocervical junction, and, most importantly, the ability to view the brain in any plane. MR imaging has, for all practical purposes, completely replaced CT for this indication. The one exception is in evaluation of osseous structures including various craniofacial anomalies and in suspected premature fusion of the cranial sutures.
CT is the preferred modality for studying practically all acute head injuries. Examination times are short, intracranial hemorrhage is well demonstrated, and skull fractures are readily apparent. Unstable patients can also be easily monitored. Intravenous administration of contrast agents is unnecessary in the usual trauma setting. CTA and occasionally MRA are utilized with increasing frequency to assess for vascular injury associated with blunt or penetrating trauma. CTA is typically the first-line evaluation for dissection or laceration, particularly when a displaced fracture crosses a vascular foramen or in the case of penetrating vessel injury. Occasionally, cerebral arteriography is performed to look for carotid or vertebral artery injury, particularly when CTA or MRA are inconclusive or when there is an anticipated endovascular treatment of the injured vessel.
Although MR imaging is not routinely performed in the acute trauma setting, it may sometimes be helpful in patients with neurologic deficits unexplained by a head CT examination. For example, traumatic brainstem hemorrhages are often difficult to see on CT scans but are usually quite obvious on MR images. MR imaging is also useful in demonstrating tiny shear lesions within the brain in diffuse axonal injury and in assessing the brain in remote head trauma.
The best examination to perform in most cases of suspected acute intracranial hemorrhage is a head CT scan. CT scans can be obtained quickly, allowing rapid initiation of treatment, and they are very good at demonstrating all types of intracranial hemorrhage, including subarachnoid blood. Because most nontraumatic subarachnoid hemorrhage (SAH) is secondary to a ruptured cerebral aneurysm, CTA is now performed routinely following a conventional head CT demonstrating SAH. In most cases, the CTA is adequate for aneurysm detection and characterization prior to surgical or endovascular treatment. MR imaging takes much longer to perform in a potentially unstable patient, and subarachnoid hemorrhage may be difficult to see. However, MR imaging is more useful in the subacute or chronic setting, especially because it gives information about when a hemorrhagic event occurred. This information might be useful in such settings as nonaccidental head trauma (eg, child abuse). MR imaging is also very sensitive to petechial hemorrhage that frequently accompanies a cerebral infarction and could potentially help to identify an underlying cause for an intracranial hemorrhage (eg, tumor, arteriovenous malformation, occluded dural sinus). Cerebral arteriography is generally reserved when the etiology of hemorrhage is not discernable by CTA/MRA, when it is necessary to evaluate the flow dynamics of a vascular lesion or for planning endovascular treatment.
Although cerebral arteriography has traditionally been considered the “gold standard” for cerebral aneurysm evaluation, CTA has supplanted catheter arteriography as the first-line imaging modality for aneurysm detection. The current literature varies slightly; however, CTA is reported to have excellent sensitivity (greater than 95% for aneurysms measuring 4 mm or larger) as well as high specificity. In most cases, CTA is adequate for surgical or endovascular treatment planning. If CTA fails to identify a suspected aneurysm following SAH, cerebral arteriography will typically be performed. Cerebral arteriography not only allows aneurysm identification, but also provides other critical preoperative information such as aneurysm orientation, presence of vasospasm, location of adjacent vessels, and collateral intracranial circulation. Arteriography also helps to determine which aneurysm has bled when more than one aneurysm is present. As mentioned previously, interventional neuroradiologists can treat aneurysms, usually in nonsurgical patients, by placing thrombosing material (ie, coils) within the aneurysm itself via an endovascular approach.
Although most patients with symptomatic cerebral aneurysms present with subarachnoid hemorrhage, some aneurysms act like intracranial masses. These situations usually warrant evaluation by MR imaging as a first examination. The same is sometimes true with posterior communicating artery aneurysms (which can produce symptoms related to the adjacent third cranial nerve) or with aneurysms arising from the internal carotid artery as it courses through the cavernous sinus (which can affect any of the cranial nerves that lie within this structure, including cranial nerves III, IV, V, or VI).
Patients with a vascular malformation (eg, arteriovenous malformation, cavernous angioma, venous angioma, or capillary telangiectasia) often seek medical attention after an intracranial hemorrhage or a seizure. In this setting, the first test that should be performed is either a CT examination (to look for intracranial hemorrhage) or MR imaging. Although an intracranial hemorrhage is usually very obvious on a CT scan, the vascular malformation itself may be difficult, if not impossible, to see unless intravenous contrast material is administered. MR imaging, on the other hand, is quite sensitive for detecting vascular malformations, whether they have bled or not. The choice of the initial examination for evaluation of a vascular malformation can be difficult. Usually, patients undergo noncontrast head CT scanning to look for intracranial hemorrhage when they come to the emergency department. This is usually followed by CTA, particularly if an arteriovenous malformation (AVM) is suspected. Otherwise, the head CT is followed by gadolinium-enhanced MR imaging to further characterize the CT findings. If a true high-flow arteriovenous malformation is suspected, either clinically or from a cross-sectional imaging study, then cerebral arteriography is performed. In contrast to cerebral aneurysms, catheter angiography is still performed routinely to evaluate AVMs. This is done because catheter angiography provides details of flow dynamics within the AVM and demonstrates certain anatomic features that are necessary to elucidate prior to initiation of treatment. As spatial resolution and dynamic sequences improve, CT or MR angiography may someday replace conventional arteriography in the workup of these lesions, as with aneurysms.
Today, most patients with suspected cerebral infarction undergo CT scanning in the acute setting, even though infarctions are demonstrated earlier and are more obvious on MR imaging. So why is CT usually performed first? The answer is that clinicians who manage stroke patients are not so interested in seeing the infarct itself. Infarct location is usually suspected from the physical examination, and acute infarcts may not even be visible on CT scans for 12 to 24 hours after onset of stroke symptoms. Clinicians are very interested, though, to know if a stroke is secondary to something besides an infarct (eg, intracranial hemorrhage, brain tumor), or if an infarct is hemorrhagic, because thrombolytic agents would be contraindicated in this setting. CT can quickly answer both of these questions. MR imaging, specifically diffusion-weighted imaging, can sensitively detect acute infarctions and is typically ordered in cases of high clinical suspicion, when the initial CT study is nondiagnostic or when brainstem or posterior fossa infarcts are suspected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree