Abstract
Purpose
The aim of this study was to report the safety and tumor response rate of combined transarterial radioembolization (TARE) through the intrahepatic arteries and transarterial chemoembolization (TACE) through the extrahepatic feeding arteries (EHFA) in patients with hepatocellular carcinoma (HCC).
Methods
Patients with HCC, who had both intrahepatic and extrahepatic arterial supply visible on preinterventional multiphase CT and were treated between 2016 and 2021 with a combination of TACE and TARE on the same nodule, were retrospectively included. Epidemiological, clinical, biological, and radiological characteristics were recorded. Safety and tumor response were assessed at 6 months.
Results
Nine patients (8 men, median age 62 years [IQR: 54–72 years]) were included. Seven patients had previous treatments on the target nodule (TARE: 5; TACE: 2). The median longest axis (LA) of the lesion was 70 mm (IQR: 60–79 mm). Three patients had portal vein invasion (VP3). The EHFA originated from the right diaphragmatic artery ( n = 6), the right adrenal artery ( n = 2), and the left gastric artery ( n = 1). The LA of the tumor portion treated with TACE was 47 mm (range: 35–64 mm). The ratio between the LA of the entire lesion and the LA treated with TACE was 1.44 (range: 1.27–1.7). One major complication occurred: acute on chronic liver failure. Median follow-up was 23 months (range: 16–29 months). Seven patients underwent further treatment: on the same lesion ( n = 2), on newly appeared nodules ( n = 2), and systemic treatment ( n = 3). At 6-month follow-up, seven patients showed a local objective response. Time-to-progression was 13 (3.5–19) months.
Conclusion
The combination of TARE and extrahepatic TACE for HCC with both intrahepatic and extrahepatic arterial supplies seems feasible and safe. Further studies are needed to validate the effectiveness of these preliminary results.
1
Introduction
Transarterial radioembolization (TARE) was included in the 2022 Barcelona Clinic Liver Cancer (BCLC) guidelines as a therapeutic option for patients classified as BCLC stages 0 and A. This recommendation specifically applies to cases where ablation or resection is not feasible and only for lesions smaller than 8 cm in their longest axis. Furthermore, according to recommendations from various national and international societies, such as the European Society for Medical Oncology (ESMO) or the National Institute for Health and Care Excellence (NICE) , TARE also plays a role in the palliative treatment of patients classified as stage B or C according to the BCLC classification. This application of TARE is particularly relevant for patients without extrahepatic lesions who have contraindications to systemic therapy or transarterial chemoembolization (TACE). The technique itself does not differ from TACE, but, as the local tumor control depends on a high dose of radiation delivered to the tumor, selective catheterization of the arteries feeding the tumor is needed to minimize the risk of non-target damage to the surrounding liver parenchyma . Moreover, as TARE can be proposed as a salvage therapy for lesions over 5 cm in diameter, most patients have already had several previous treatments: i.e., surgery, systemic treatment, ablations, or TACE . Thus, lesions may have arterial abnormalities induced by tumor progression or previous embolizations, leading to the recruitment of extrahepatic feeding arteries (EHFA), especially in the case of peripherally located tumors . However, TARE cannot be performed through an EHFA as the risk of delivering radiation to non-target territories is elevated and burdened by major complications . Thus, embolization of EHFAs has been proposed to redistribute arterial flow to the liver lesion , facilitating effective redistribution of the radiation dose during TARE performed solely through the hepatic arteries. Essentially, two techniques have been proposed: coiling or embolization with particles of the EHFA [ , , ].
The first option was described as being ineffective due to the opening of shunts around the EHFA [ , ]. Conversely, the second option seems reliable in redistributing the flow to the intrahepatic branches, but it risks occluding the vascularization to a part of the lesion which consequently will not be treated during TARE and thus not receive the appropriate radiation dose.
Herein, we report a series of patients affected by HCC with EHFAs who underwent TARE through the intrahepatic vessels and TACE through the EHFA in order to evaluate the safety, feasibility, and tumor response rate of the combined treatment.
2
Materials and methods
This is an observational, single-institution, retrospective study concerning patients with HCC treated with concomitant TARE and TACE through intra- and extrahepatic approaches between March 2016 and March 2021.
Informed consent was obtained from every patient, and medical ethics approval was waived due to the retrospective nature of the study.
HCC was diagnosed either by biopsy or based on imaging characteristics, following the guidelines of the European Society for the Study of the Liver (EASL) .
The indication for TARE was given after a multidisciplinary meeting involving hepatologists, oncologists, surgeons, and radiologists. The decision was based on the non-resectability of the lesion due to its dimensions, position, or clinical risks associated with surgical treatment.
A contrast-enhanced CT scan (CECT) is routinely performed at our institution for patients selected for endovascular treatment of HCC. EHFAs were identified, paying particular attention to tumors in contact with the diaphragm or near the right or inferior border of the liver, due to the possibility of vascularization through the phrenic, intercostal, adrenal, or lumbar arteries as previously described in the literature If an EHFA supplying the lesion was not found at the preliminary CECT, its presence could be suspected on the yttrium 90 ( 90 Y) PET/CT obtained after TARE, in cases where a subcapsular circumscribed area lacked microsphere distribution. In these cases, TACE was performed in a subsequent session. Patients were included if they had TARE and TACE on different portions of the same lesion, either in the same session or within a 3-month interval.
Demographic and clinical characteristics were obtained for the selected population, including age, gender, cause of liver cirrhosis (if any), tumor morphology, and previous liver treatments.
BCLC, Child-Pugh, and Eastern Cooperative Oncology Group (ECOG) scores , as well as laboratory parameters (i.e., alpha-fetoprotein [AFP], serum albumin, prothrombin time [PT], aspartate transaminase [AST], alanine transaminase [ALT], and total bilirubin) were collected before treatment and during follow-up. BCLC was recalculated based on the version updated in 2022 .
2.1
TARE
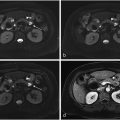
All treatments were performed by a senior radiologist with at least 2 years of experience in endovascular procedures. TARE was performed in two sessions, as per the conventional model, with a workup phase and a treatment phase. The workup aimed to find the exact point of injection to cover the whole lesion in the treatment field while minimizing non-tumoral parenchyma [ Fig. 1 ]. The correct positioning of the microcatheter was always confirmed with a dual-phase cone-beam computed tomography (DP-CBCT), and followed by a slow injection of technetium ( 99 mTc) albumin aggregated ([ 99 mTc]-MAA). The patient then underwent a SPECT/CT within 1 h after the workup to check for any extrahepatic diffusion of ( 99 mTc)-MAA and to confirm the coverage of the entire target lesion in the injection area.

During the same session, an angiography was performed through the EHFA to confirm its contribution to the HCC vascularization.
The treatment was carried out within 14 days using 90 Y, following the same injection pattern as in the workup. The delivered radiation dose was recorded.
When combined with TARE, TACE was performed during the same treatment session.
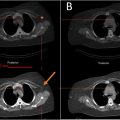
A 90 Y PET/CT was performed within 24 h to assess the distribution of yttrium 90 [ Fig. 2 ].

2.2
TACE
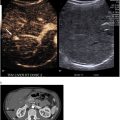
TACE was executed after catheterization of the EHFA [ Fig. 3 ]. A DP-CBCT was conducted before injection to identify collateral branches not contributing to tumor vascularization. If any were found, they were excluded by coil embolization. The treatment consisted of an emulsion of Lipiodol (10 ml) (Guerbet, France) and doxorubicin (60 mg/9 ml) (1:1) injected until flow stasis or the emulsion was exhausted [ Fig. 4 ]. The embolization was completed with the injection of Embozene 400 μm (Varian Medical Systems, Palo Alto, CA). A non-enhancement CBCT was performed at the end of the procedure to confirm the correct impregnation of the part of the lesion vascularized by the EHFA. All procedures were performed with Allura Clarity and Azurion (Philips, Best, Netherlands).


The volume and main diameter of the part of the lesion treated with TACE were calculated using hyperdensity due to Lipiodol captation on the CBCT obtained at the end of the procedure, and compared to the volume and main diameter of the whole lesion, respectively.
The feasibility of the procedure, defined as the ability to perform a complete TARE and a complete TACE on the same target lesion, was evaluated.
Complications of the procedures were recorded and classified using the SIR scale for adverse events . Safety, defined as the possibility of carrying out the procedure without major complications (i.e., class C-F of the SIR Classification), was also evaluated.
2.3
Follow-up
Follow-up was conducted with clinical and imaging evaluations (i.e., MRI or CT scan) one month after the treatment and then every three months. The median duration of follow-up was recorded.
Objective response (OR) of the target lesion was evaluated at 6 months, using mRECIST criteria . Time-to-progression was recorded, as well as any further local or systemic treatment performed after TARE.
2.4
Statistics
Due to the non-Gaussian distribution, descriptive statistics were used as appropriate, including proportions and medians with quartiles (first and third quartile).
3
Results
Between March 2016 and March 2021, 13 patients with HCC underwent TARE associated with TACE. Four patients were excluded because TACE was performed more than three months after TARE.
Individual tumor dimensions, the presence of portal vein invasion, as well as main clinical and laboratory status at baseline are reported in Table 1 . Of the nine remaining patients, there were eight men and one woman, with a median age of 62 years (IQR: 54–72 years). HCC was diagnosed by biopsy in two cases and based on imaging characteristics in seven cases. Cirrhosis was present in seven cases, developing from alcohol abuse in five cases, hepatitis B virus (HBV) in two cases, and hepatitis C virus (HCV) in two cases. Alcohol abuse was associated with HCV in one case and with hemochromatosis in another case. At the clinical evaluation before TARE, six patients were classified as BCLC stage A and three patients as BCLC stage C.
| Liver disease | Tumor volume (cm3) | Lipiodol volume in the tumor (cm3) | Portal vein invasion | Previous treatment on the same nodule | AFP | Clinical status | Laboratory | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCLC | Child Pugh | ECOG | Albumine | PT | Bilirubin | |||||||
| Patient 1 | HCV | 213 | 60 | VP 3 | TACE | 21N | C | A5 | 0 | N | N | N |
| Patient 2 | OH | 260 | 75 | VP 3 | TARE | 8N | C | A5 | 1 | N | N | N |
| Patient 3 | OH | 82 | 11 | VP 0 | TARE | N | A | A5 | 0 | N | N | N |
| Patient 4 | HCV | 75 | 40 | VP 0 | TARE | 64N | A | A5 | 0 | N | N | N |
| Patient 5 | HBV | 11 | 5 | VP 0 | TARE | 485N | A | A5 | 0 | N | N | N |
| Patient 6 | HBV | 200 | 24 | VP 0 | N | A | A5 | 0 | N | N | N | |
| Patient 7 | OH/Hemocromatosis | 300 | 75 | VP 3 | TARE | 31N | C | A5 | 0 | N | N | N |
| Patient 8 | OH | 2500 | 110 | VP 0 | TACE | N | A | A5 | 0 | N | N | N |
| Patient 9 | OH/HCV | 67 | 20 | VP 0 | 11N | A | B8 | 0 | 5 < N | 5 < N | 2N |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree