Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States. Most cases arise from polyps, which can be detected and removed before becoming cancerous. Computed tomography colonography (CTC), also known as virtual colonoscopy, was first introduced in 1994 as a minimally invasive method for CRC screening and diagnosis. This 2025 update on CTC will focus on (1) techniques and dose reduction strategies, (2) image display methods, (3) reporting and classification systems, (4) tumor staging capabilities, (5) integration of advanced imaging techniques, and (6) cost-effectiveness and reimbursement.
Key points
- •
Computed tomography colonography has now been around for 3 decades and has evolved significantly since it was first introduced in 1994.
- •
Numerous improvements and refinements in the technique have been developed, standardized, and adopted leading to clinical performance comparable to optical colonoscopy.
- •
Updates in bowel preparation, scanning techniques, image display, and reporting systems.
- •
Advances in tumor staging and integration of radiomics.
- •
Cost-effectiveness and reimbursement progress.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths in the United States, responsible for approximately 150,000 new cases per year and 1 in 11 deaths or approximately 53,000 deaths per year. , The average American has a 4% to 5% risk of developing colon cancer at some point in their lifetime. Because of the increased use of colorectal screening, CRC incidence and mortality has significantly decreased overall since the 1980s. However, in those under the typical age for colorectal screening, incidence and mortality have significantly increased, recently becoming the leading cause of cancer death in male individuals aged under 50 years and the second leading cause in female individuals aged under 50 years with global rates continuing to rise rapidly.
Most CRCs arise from polyps that can be detected and removed before becoming cancerous. Computed tomography (CT) colonography (CTC), also known as virtual colonoscopy, was first introduced in 1994 as a noninvasive alternative to traditional colonoscopy for CRC screening and diagnosis. The accuracy of CTC in cancer detection as well as its speed in acquisition and quality has improved significantly since its introduction, driven by advancements in technology, techniques, and increased clinical experience. The introduction of multidetector CT significantly improved image resolution and acquisition speed, leading to improved polyp detection. Furthermore, advances in 3 dimensional (3D) imaging software allowed for more accurate and efficient interpretation of images.
In the late 2000s and 2010s, several large-scale, multicenter trials demonstrated the efficacy of CTC. In a pivotal study by Pickhardt and colleagues, CTC showed a sensitivity of 0.94 for polyps 10 mm or greater, 0.94 for polyps 8 mm or greater, and 0.89 for polyps 6 mm or greater, with specificities of 0.96, 0.92, and 0.80, respectively. In validation, the American College of Radiology Imaging Network (ACRIN) National CT Colonography trial led by Johnson and colleagues found that CTC identified 90.0% of patients with large adenomas or cancers (≥10 mm) detected by optical colonoscopy (OC). The sensitivity for lesions 10 mm or greater was 0.90, with a specificity of 0.86. Additional international validation trials including the Munich Colorectal Cancer screening trial and Italian Multicenter Polyps Accuracy CTC (IMPACT) trial in Italy also confirmed sensitivities of 92.0% and 91.0% for 10 mm and larger polyps, respectively. , Finally, CTC resulted in fewer polypectomies and complications, supporting its use as a primary screening test before therapeutic optical colonoscopy.
Since the early 2010s, integration with artificial intelligence and computer-aided detection (CAD) systems has been studied to increase diagnostic accuracy by assisting in identifying suspicious lesions. Also, advances in CT technology have allowed for significant reductions in radiation exposure without compromising image quality. CTC has since been included in CRC screening guidelines by several major medical organizations, reflecting its established accuracy and utility. ,
This update on CTC focuses on (1) updates in bowel preparation, scanning techniques, and dose reduction methods; (2) enhancements in image display; (3) changes in reporting and classification systems; (4) advances in tumor staging and integration of advanced imaging techniques; and (5) cost-effectiveness and reimbursement progress.
Bowel preparation, scanning techniques, and dose reduction methods
Bowel Preparation
Effective colon preparation is critical for the success of CTC, as it ensures clear visualization of the colonic mucosa and accurate detection of polyps and other abnormalities.
Cleansing
This involves the use of dietary restrictions and cathartic agents to cleanse the colon. Patients follow a clear liquid diet for at least 24 hours before the examination, avoiding solid foods. Fluids of specific colors that might be mistaken for blood can also be omitted if same-day diagnostic optical colonoscopy is being offered for positive scans. As for catharsis, there are 2 major categories: (1) high-volume iso-osmolar cathartics are usually polyethylene glycol-based regimens. Although effective, the higher 2 to 4 L volume may be more difficult for patients to tolerate, and the larger residual fluid volume may make colonic distension and polyp visualization more difficult. (2) Low-volume hyperosmolar cathartics such as magnesium citrate and sodium picosulfate are preferred for better patient tolerance and to leave less residual colonic fluid to aid 3D visualization and polyp detection.
Evolving bowel preparation practices include the use of split-dosing , which involves taking half of the laxative dose the evening before and the other half the morning of the examination, which has been shown to be sufficient for bowel cleanliness and improve patient tolerance. ,
Other emerging alternatives to traditional bowel preparation methods include the use of noncathartic preparations or minimal preparation regimens that use oral contrast agents without extensive laxative consumption, improving patient compliance and comfort. Although some studies have shown that reducing the quality of bowel preparation has detrimental effects on CTC performance, this may be a viable option when patients cannot or will not tolerate a full-laxative bowel preparation such as certain elderly or frail populations where the primary goal of CTC is the detection of malignancy rather than of precancerous polyps.
Fecal/fluid tagging
Contrast tagging of residual stool and fluid is a technique used to improve the differentiation between residual fecal material or fluid and colonic lesions by increasing the attenuation of residual fluid and stool, improving visualization of submerged polyps that remain of soft tissue attenuation.
Tagging agents, such as iodinated contrast and barium sulfate, are ingested during the bowel preparation process and can be ingested either the night before or as a split dose the night before and the morning of the CTC. Iodinated contrast agents (eg, diatrizoate, iohexol, and iopamidol) are effective for fluid tagging, while barium tends to be better for stool tagging. Oral iodinated contrast is recommended at a minimum while a combination of both iodine and barium may be preferred for more comprehensive tagging as well as to facilitate polyp coating, a phenomenon increasingly recognized in certain flat and serrated lesions. ,
Bowel distension
Adequate colonic distension is essential for clear visualization. Automated carbon dioxide insufflation is preferred over manual air insufflation for more consistent and higher volume distension, leading to a shorter examination to improve patient comfort and reduce the risk of perforation. At least 2 different patient positions (eg, supine and prone, supine and lateral decubitus, and so forth) help redistribute gas and residual fluid, maximizing visualization of the colonic mucosa. An optional third decubitus position should also be utilized to scan any specific colonic segments that may have not fully distended on the initial 2 positions.
Scanning Techniques and Dose Reduction Methods
Concerns over radiation risks associated with CTC have been raised by patients, physicians, and regulatory agencies. The theoretic risks of low-dose radiation from CTC are minimal compared to the benefits of CRC screening. Recent advancements in CT technology continue to reduce radiation dose in CTC while preserving image quality. Strategies and techniques to minimize radiation exposure during CT are aligned with the as low as reasonably achievable (ALARA) principle and include CT scan parameter adjustments and other practical methods explained in later discussion.
Computed tomography scan parameters
Modern CT scanners with multidetector arrays are sufficient for CTC. Scanning involves at least 2 patient positions (such as supine and prone) to ensure comprehensive visualization. Typical parameters include 0.625 to 1.25 mm collimation and reconstruction intervals, acquired in a single breath-hold. Thin slice imaging improves sensitivity and specificity for polyps.
Reducing tube current (milliampere-seconds) reduces radiation dose linearly but increases image noise; however, CTC can tolerate more noise compared to other CT examinations as only a low dose is needed to accurately render the gas–mucosal interface, similar in principle to dose reduction for renal stone detection and pulmonary nodule screening. Automatic dose modulation technique intelligently adjusts the tube current during a single scan based on patient anatomy and x-ray beam attenuation, reducing the dose in less dense areas and in thinner patient projections without compromising image quality. Reducing tube voltage (kilovoltage peak) significantly reduces radiation dose but also increases image noise; it is an approach more beneficial in smaller patients. Finally, advanced image reconstruction methods such as iterative reconstruction and deep learning-based image reconstruction can mitigate image noise, allowing for lower radiation doses while improving image quality. ,
Patient isocentering , or ensuring the patient is properly centered in the CT gantry, optimizes image quality and the impact of automatic dose modulation. Furthermore, minimizing scan phases such as by limiting the number of scans and the scan volume to only the colon further reduces unnecessary radiation exposure.
Careful implementation and monitoring are essential to maintain clinical accuracy. Measuring CT radiation dose metrics such as CT dose index (CTDIvol) and dose length product is critical for monitoring and minimizing radiation exposure . Furthermore, comparing radiation doses across institutions can help establish benchmarks and improve dose reduction strategies such as through registries like the American College of Radiology (ACR’s) National Radiology Data Registry.
Image display
Familiarity with various display methods and advancements in software tools can lead to more efficient and accurate CTC interpretations, ultimately improving CRC screening outcomes. The use of both 2 dimensional (2D) and 3D visualization modes is vital for polyp detection and characterization. Primary 3D endoluminal evaluation is emphasized for its higher polyp sensitivity, with 2D evaluation used to confirm lesion attenuation.
Primary 2 Dimensional Interpretation
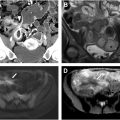
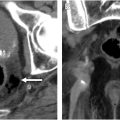
It involves initially evaluating axial and multiplanar reformatted CT images and then secondarily correlating findings with the 3D view for morphology and size measurement ( Fig. 1 , bottom left, top left, and top right). Advantages include familiarity for radiologists, better visualization of colonic wall thickness, and easier detection of extracolonic findings. Disadvantages include the potential for greater interobserver variability and reader fatigue.

Primary 3 Dimensional Interpretation
It involves initial interactive 3D endoluminal fly-throughs with secondary correlation with the source axial 2D CT images to confirm polyp attenuation and to help visualize any submerged polyps and incompletely distended segments. Endoluminal Views are advanced software algorithms that generate highly detailed and realistic 3D representations of the colon’s interior, closely mimicking the perspective of traditional optical colonoscopy (see Fig. 1 , bottom right). These endoluminal views improve navigation and polyp detection. Virtual Fly-Through is a dynamic visualization variant that simulates a virtual journey through the colon, enabling radiologists to inspect the mucosa in a manner similar to optical colonoscopy.
Novel Display Methods
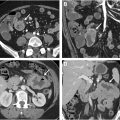
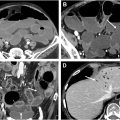
Advances in image display include virtual dissection, pathology, or filet views , which involves unfolding and flattening the colon for a comprehensive view of the mucosal surface, reducing evaluation time but introducing potential distortion ( Fig. 2 , top right). Unfolded cube projection displays the entire panorama of the colonic lumen in 6 projections as an unfolded box, providing a 360° fly-through field of view and reducing interpretation time ( Fig. 3 ).


Electronic Cleansing and Computer-Aided Detection
These tools facilitate image interpretation and maximize reader performance. When used together, they have the potential to significantly reduce interpretation times. Modern CAD systems incorporate sophisticated algorithms to automatically identify and highlight potential polyps, reducing the likelihood of human error, particularly for beginning readers, and increasing the overall sensitivity of CTC although they may add additional interpretation time and reduce specificity if there are too many false positives. CAD systems are now more seamlessly integrated into the radiologist’s workflow, allowing for real-time analysis and immediate feedback during the reading of CTC scans. Deep learning algorithms have also been recently employed to improve the electronic subtraction of contrast-tagged fluid and fecal material as well as to attempt differentiation of benign from premalignant polyps. ,
Reporting and classification
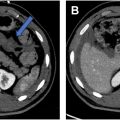
The CT Colonography Reporting and Data System (C-RADS) was established by the ACR to standardize the reporting and management of findings from CTC. The second oldest ACR RADS classification system, first introduced in 2005, has been pivotal in guiding the interpretation and communication of both colonic and extracolonic findings.
C-RADS was updated in 2023 and introduced several significant changes aimed at improving clarity and management recommendations as well as reducing unnecessary follow-ups for clinically insignificant findings. It enhances the consistency of reporting across different centers with the use of standardized terminology and lesion classification to ensure consistent patient management and follow-up. Furthermore, by introducing more specific subcategories and simplifying the categorization of extracolonic findings, the updated system is expected to reduce ambiguity in reports and reduce the overdiagnosis of clinically insignificant extracolonic findings. Finally, the standardized classification provided by C-RADS facilitates the collection of uniform data, which can be used in research studies as well as compare quality metrics and patient outcomes through evidence-based practices ( Figs. 4 and 5 ).