Congenital pulmonary vascular disease is a daunting and diverse topic spanning both pulmonary arterial and venous anomalies. Given advancements in treatment, patients with congenital anomalies have longer life expectancies into adulthood and practicing radiologists are bound to come across these patients during their daily practice. Additionally, many anomalies are discovered incidentally on imaging, yet may still have implications for patient care. This article provides an overview of entities most likely encountered in adulthood, highlighting key pre-operative and post-operative imaging features and relevant clinical considerations.
Key points
- •
Congenital pulmonary vascular anomalies and diseases encompass a wide range of entities involving the pulmonic valve, pulmonary arteries, and/or pulmonary veins, many of which occur in the setting of additional congenital anomalies.
- •
Given the advancements in treatments, these patients have longer life expectancies, and therefore familiarity with these entities, including pre-operative and post-operative imaging and possible post-repair complications are imperative.
- •
Some entities are asymptomatic or variable in their presentation/onset and therefore radiologists may be the first to discover.
- •
Contrast-enhanced cross-sectional imaging, whether with computed tomography angiography or magnetic resonance angiography, should be considered the first-line modality in these adult patients and is generally sufficient to make the diagnosis, problem-solve, and perform follow-up evaluation.
Introduction
Congenital pulmonary vascular anomalies and diseases encompass a wide range of entities. This article will focus on those that are most likely encountered in adulthood, highlighting key imaging features and relevant clinical considerations. Although many occur in the setting of concurrent cardiac anomalies, the authors’ discussion will be focused on the manifestations in the pulmonary arteries and veins. A list of entities discussed is outlined in Box 1 .
Pulmonary Arterial Anomalies
Conotruncal Anomalies
- •
Tetralogy of Fallot
- •
Pulmonary atresia
- ○
Pulmonary atresia with ventricular septal defect
- ○
Pulmonary atresia with intact ventricular septum
- ○
- •
Truncus arteriosus
- •
Other Pulmonary Arterial Anomalies
- •
Patent ductus arteriosus
- •
Pulmonary artery stenosis
- ○
Valvular stenosis
- ○
Subvalvular stenosis
- ○
Supravalvular stenosis
- ○
- •
Pulmonary sling
- •
Proximal interruption of the pulmonary artery
- •
Crisscross or crossed pulmonary arteries
- •
Pulmonary arteriovenous malformation
- •
Pulmonary Venous Anomalies
- •
Normal variants
- •
Total anomalous pulmonary venous return
- •
Partial anomalous pulmonary venous return
- ○
Scimitar syndrome
- ○
- •
Levoatriocardinal vein
- •
Meandering pulmonary vein
- •
Varix
- •
Pulmonary arterial anomalies
Conotruncal Anomalies
The following section will focus on the relevant findings pertaining to the pulmonic valve, main pulmonary artery (MPA), and branch pulmonary arteries in select congenital heart diseases (CHD).
Tetralogy of Fallot
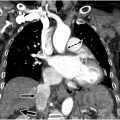
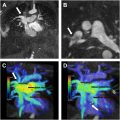
Tetralogy of Fallot (TOF) is the most common cyanotic congenital disorder accounting for 7% to 10% of all CHD. Anterior malposition of the conal septum gives rise to the 4 classic features: right ventricular outflow tract (RVOT)/pulmonary artery (PA) obstruction, overriding aorta, outlet/membranous ventricular septal defect (VSD), and consequent right ventricular (RV) hypertrophy. , The severity and onset are mainly determined by the degree of RVOT/pulmonary artery obstruction, which can range from mild RVOT obstruction (with or without pulmonic valvular stenosis) to pulmonary atresia. When there is severe pulmonary stenosis or atresia, pulmonic blood flow may be dependent on a patent ductus arteriosus (PDA) or major aortopulmonary collateral arteries (MAPCAs). The location of the pulmonary outflow tract stenoses/obstruction is variable and can affect the branch pulmonary arteries ( Fig. 1 A–C ).

Corrective surgery involves VSD closure and relieving the RVOT/pulmonary obstruction. Typically, a transannular patch made of autologous pericardium, Dacron, or Gore-Tex is used to augment and increase the diameter of the RVOT (see Fig. 1 ). The patch can also be extended to the main and/or branch pulmonary arteries to increase their diameter.
Computed tomography (CT) and MRI are both helpful in the pre-operative and post-operative assessment. Post-operative imaging can reveal VSD patch leaks, RVOT obstruction, residual/recurrent branch pulmonary artery stenosis, and aortopulmonary collaterals. Approximately 50% of repaired TOF will undergo reoperation within 30 years for pulmonary valve replacement due to valvular dysfunction, most commonly pulmonary regurgitation.
Pulmonary atresia
Pulmonary atresia is a lack of communication between the RV and the pulmonary arteries either due to muscular atresia (overgrowth of the infundibulum and RV apex) or from valvular atresia. It can be divided into those with a ventricular septal defect (PA-VSD) and those with an intact ventricular septum (PA-IVS).
Pulmonary atresia with ventricular septal defect
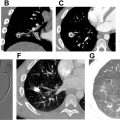
In pulmonary atresia with ventricular septal defect (PA-VSD), often considered a severe form of TOF, the main and branch pulmonary arteries are often atretic or hypoplastic and may be discontinuous with one another ( Fig. 2 A–E ). This necessitates the need for extracardiac sources of pulmonary blood flow, usually a PDA or MAPCAs. PA-VSD can be classified into 3 types based on the source of pulmonary blood supply:
- 1.
Type A with blood supply from the native pulmonary artery
- 2.
Type B with blood supply from both native pulmonary artery and MAPCAs
- 3.
Type C with MAPCAs only

MAPCAs are large arterial collateral vessels which typically arise from the descending thoracic aorta but can also arise from the aortic arch and arch vessels, abdominal aorta, or more rarely, from other systemic arteries such as the coronary arteries. MAPCAs then anastomose with the pulmonary arteries in the mediastinum and hila at the lobar or subsegmental level to provide blood flow into the lungs (see Fig. 2 ). , PDA and MAPCAs will typically not supply the same side of the lung. PDA can supply one or both branch pulmonary arteries.
Management can be broadly divided into rehabilitation, unifocalization, or a combination of both. Rehabilitation is aimed at creating a shunt to promote growth of the native pulmonary arteries allowing later definitive repair with an extracardiac RV-to-PA conduit. , Unifocalization is aimed at ligating, mobilizing, and anastomosing the ipsilateral MAPCAs to each other and incorporating them into the native pulmonary artery.
CT or MRI is the key for delineating the pulmonary arterial anatomy to direct the repair approach. The following should be reported:
- •
Level at which the atresia occurs
- •
Degree of the hypoplasia
- •
Connections between the MPA and the branch arteries
- •
Number, size, origin, and course of MAPCAs
- •
Extracardiac sources of blood flow
In the post-operative assessment, special attention should be made to the main and branch pulmonary arteries (for stenoses) and MAPCAs (for stenoses, aneurysm, or associated pulmonary hemorrhage).
Pulmonary atresia with intact ventricular septum
In the absence of a VSD, PA-IVS requires an alternative blood flow route to the pulmonic circulation—generally via a patent foramen ovale (PFO) or atrial septal defect (ASD) and then to pulmonary arteries via a PDA. Patients are therefore PDA dependent and present earlier with cyanosis in the first 48 hours of life as the ductus arteriosus closes. In contradistinction to PA-VSD, the RV is often hypoplastic and small as the absence of shunting inhibits RV cavity growth.
PA-IVS is associated with coronary artery anomalies, including ventriculocoronary sinusoids/fistulae and coronary stenosis/atresia. Ventriculocoronary sinusoids/fistulae are intramyocardial channels that directly connect the coronary artery to the ventricular cavity while bypassing a capillary bed (see Fig. 2 ). As a result, the coronary system becomes RV flow dependent and elevated RV flows must be maintained to provide adequate coronary perfusion, which can affect the surgical approach for repair.
Truncus arteriosus
Truncus arterious (TA) is an overriding common arterial trunk with a single semilunar valve that gives rise to both the aorta and at least 1 pulmonary artery thus supplying the systemic, pulmonic, and coronary circulation. , There are multiple variations depending on the origins and branching pattern of the pulmonary arteries. For example, the MPA can arise from the common trunk, branch pulmonary arteries can be discontinuous and arise directly from the common trunk with separate ostia, or one branch can arise from the trunk while the other is supplied by a PDA or MAPCAs. TA can be associated with a host of cardiovascular anomalies including VSD, interrupted arch, and anomalous pulmonary venous return, as well as genetic syndromes, most commonly DiGeorge syndrome.
TA is diagnosed in the neonatal period and typically surgically corrected within the first few weeks of life. Corrective surgery involves separating the pulmonary artery from the common trunk and reconnecting it to the RV via a conduit, realigning the common trunk with the left ventricle (LV) to create a neo-aorta, and patch closure of the VSD. Branch pulmonary arteries and MAPCAs can be mobilized and joined to the pulmonary artery/conduit if needed ( Fig. 3 A, B ). CT or MRI is valuable in the post-operative period to evaluate for neo-aortic valve stenosis or regurgitation, RV-to-PA conduit stenosis, and VSD leak.

Other Pulmonary Arterial Anomalies
Patent ductus arteriosus
The ductus arteriosus is a short vessel that connects the proximal descending aorta (at the isthmus) to the MPA during fetal development. , In utero, it delivers deoxygenated blood from the MPA to the thoracic aorta. After birth, the ductus arteriosus involutes, forming the ligamentum arteriosum. If the ductus persists more than 48 hours after birth, it is considered a PDA. Most cases are sporadic, although PDAs are associated with prematurity, viral infections such as rubella, and CHD. In ductal-dependent CHD, the ductus can be maintained open using prostaglandins.
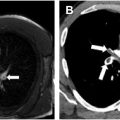
Most small PDAs are asymptomatic and incidentally found ( Fig. 4 A–D ). Moderate PDAs can become symptomatic after developing hypertension in adulthood, as the increased left-to-right shunt can lead to symptoms of heart failure. Large PDAs present in infancy to early childhood with congestive heart failure (see Fig. 4 ). If unrepaired, they can develop severe pulmonary hypertension and Eisenmenger syndrome with reversal of the flow to a right-to-left shunt.

Echocardiography is the mainstay for diagnosis in infants and children. Radiographs are typically normal in those with smaller PDAs, while larger PDAs lead to enlargement of the MPA and left-sided chambers due to over-circulation. Contrast-enhanced CT and MRI allow assessment of the PDA configuration by the Krichenko classification, useful for pre-closure planning. Phase-contrast imaging on MRI allows shunt volume and fraction quantification. , Potential pitfalls include a calcified ligamentum arteriosum (confirmed on non-contrast CT) and a ductus diverticulum (focal bulge at the level of the isthmus without connection to the MPA).
Silent or hemodynamically insignificant PDAs may not warrant any treatment. Medical management is favored in neonates. Some require surgical ligation or transcatheter closure. Surgical ligation is the primary option for premature infants or those with exceptionally large PDA and unsuitable anatomy for transcatheter closure.
Pulmonary artery stenosis
Pulmonary artery stenosis is categorized into either valvular, subvalvular, or supravalvular, in order of frequency. Most (95%) are congenital. ,
Valvular stenosis
In valvular stenosis, the pulmonic leaflets are thickened or partially fused, resulting in luminal narrowing and increased flow velocities and pressure gradients across the valve. On radiography ( Fig. 5 A–C ), CT, and MRI, there will be asymmetric enlargement of the MPA and left pulmonary artery (LPA) due to an eccentric flow jet toward the LPA leading to post-stenotic dilation (see Fig. 5 ). The right pulmonary artery (RPA) will be normal in caliber. ECG-gated CT or MRI allows assessment of valve morphology, and MRI allows evaluation of RV function/morphology and flow quantification across the valve with phase contrast imaging.