Consolidation, Collapse and Cavitation
This chapter discusses the radiographic patterns of pulmonary consolidation and illustrates the various pathological processes that can cause it. It also describes the features of partial and complete collapse of the major lobes of the lungs. We are all aware that it may be difficult to decide if there is abnormal parenchymal shadowing on a chest radiograph and most of us will also have missed subtle changes of lobar collapse at some stage in our careers. However, there is a systematic approach to the identification of both consolidation and collapse, and in this chapter I seek to share it. I guarantee that if the system is adopted and practised then eventually ‘pattern recognition’ will take over – in other words, ‘I have seen this pattern of abnormality lots of times before and I know what it is’. However, before any of us reaches this stage of experience, it is vital to be obsessional about following a systematic approach – but then this applies to all aspects of clinical medicine.
DEFINITIONS
Consolidation
Consolidation is a pathological term. It describes the state of the lung when alveolar gas has been replaced by fluid, cells or a mixture of the two. Various terms have been used in an attempt to describe the morphological appearance of consolidation – ‘alveolar-filling pattern’, ‘air-space filling’ and ‘ground-glass shadowing’ are examples. I prefer the first of these because it is so descriptive and I use it preferentially in this chapter.
Whatever the terminology, the radiographic appearances of consolidation are those of homogeneous shadowing in part of the lung field with little or no lobar shrinkage. The normal vascular pattern is lost because the alveolar-filling process denies the definition of lung markings by replacing the air in adjacent lung parenchyma. This loss of vascular pattern is a major clue when the appearances of consolidation are subtle.
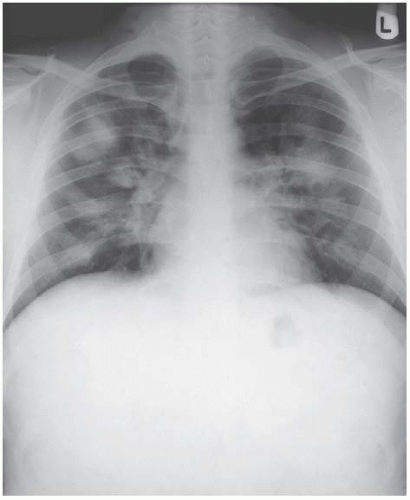
It is important to remember that none of these terms can determine the pathological nature of the substance that has resulted in the appearance of alveolar filling. An identical radiographic pattern can be the result of bacterial infection, the transudate of heart failure, alveolar haemorrhage, pneumonia due to Pneumocystis carinii or the malignant infiltrate of alveolar cell carcinoma. However, there are additional clues on a radiograph that can narrow the pathological diagnosis and one should seek these out. An example is the classical distribution of consolidation in chronic eosinophilic pneumonia. Described as ‘reverse pulmonary oedema’, the radiographic appearance is virtually diagnostic with predominantly peripheral consolidation that crosses boundaries between individual lobes and segments (Fig. 3.1).
Another example, discussed in the Preface, is an alveolar-filling pattern in association with pleural effusions, cardiomegaly and interstitial lines – a combination that virtually secures the diagnosis of heart failure (Figs 1 and 2, page viii).
Heart failure is usually associated with cardiomegaly, but not exclusively so. Normal heart size may be retained in the following circumstances:
if heart failure is of sudden onset, a classic example being mitral valve rupture after myocardial infarction
restrictive cardiomyopathy
pericardial constriction
mitral valve disease – when rheumatic heart disease was prevalent, mitral stenosis regularly progressed to cause left atrial failure, the X-ray manifestations of which are identical to left ventricular failure.
Collapse
Consolidated lung may lose volume at any stage in disease progression but the crucial question is whether consolidation is secondary to collapse. The obvious cause of this is major airway obstruction and this has important management implications. Marked loss of volume on the radiograph is likely to indicate pathology causing primary collapse and should be investigated as such.
When the primary process is consolidation, on the other hand, any subsequent loss of volume is not usually dramatic unless the disease is a chronic infective one such as tuberculosis or chronic Klebsiella pneumonia. One of the reasons for emphasizing this point is to question the value of the compromise term ‘consolidation collapse’. To use this description seems scarcely worthwhile because it does not assist in
deciding whether bronchial occlusion is present and therefore fails to materially guide patient management.
deciding whether bronchial occlusion is present and therefore fails to materially guide patient management.
Density
When referring to radiographic shadowing, the term ‘density’ refers to the radio-opacity of a lesion and this will be influenced fundamentally by the degree of exposure of the film. With this important qualification and assuming ideal radiographic exposure of the image, I think it is useful to consider three grades of density as follows:
low density – small shadows caused by cells or body fluids
medium density – larger shadows especially caused by fluids
high density – shadows containing radio-opaque atoms either derived from body fluids (iron or calcium) or introduced from the environment (iron, calcium, barium or tin).
Inevitably, these distinctions will be subjective to a certain extent and, in particular, the separation of low- and medium-density shadows can be difficult but the classification is still helpful and I would recommend it to you.
A SYSTEMATIC APPROACH TO CONSOLIDATION (OR ALVEOLAR FILLING)
Ensure that the abnormal shadowing represents an alveolar-filling process
Extensive pulmonary infiltrates of various types can coalesce and mimic ‘alveolar filling’. Examine the nature of the shadowing carefully – is it truly homogeneous or does it appear to be a coalescence of rounded shadows (nodular), streaky shadows (reticular) or a combination of the two (reticulo-nodular)? The intrapulmonary shadowing in Fig. 3.2 is certainly generalized and may appear homogeneous at first
sight but closer inspection reveals that it is made up of myriads of tiny dots in all areas of the lung fields. It almost looks as though someone has scattered the contents of a salt-cellar over the film – this is an example of military tuberculosis.
sight but closer inspection reveals that it is made up of myriads of tiny dots in all areas of the lung fields. It almost looks as though someone has scattered the contents of a salt-cellar over the film – this is an example of military tuberculosis.
What is the distribution of the abnormal shadowing?
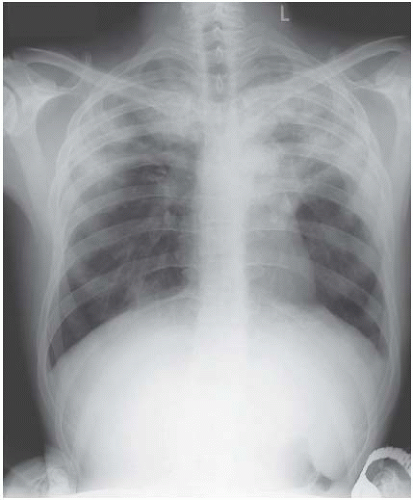
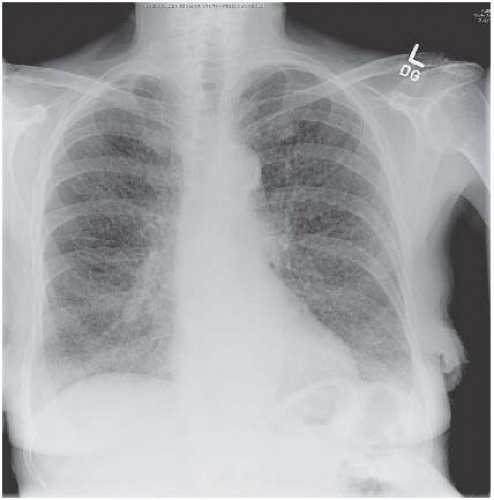
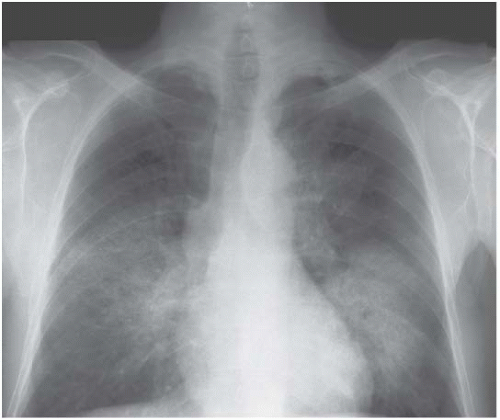
Lobar pneumonia affects lobes or segments uniformly (Figs 3.3 and 3.4). Although pneumonia caused by a variety of infecting agents, including Pneumococcus, can
affect multiple lobes or segments, the radiographic appearance of multiple segmental or subsegmental consolidation should arouse diagnostic suspicion of non-infective aetiology:
affect multiple lobes or segments, the radiographic appearance of multiple segmental or subsegmental consolidation should arouse diagnostic suspicion of non-infective aetiology:
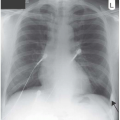
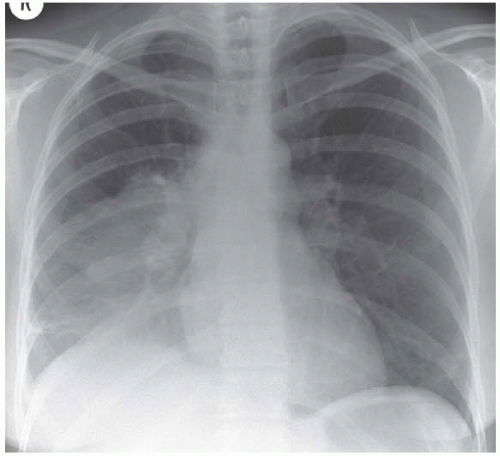 Figure 3.4 Pneumococcal pneumonia in the right lower lobe. This time the heart border is preserved, the upper boundary is indistinct and the right hemidiaphragm is blurred. |
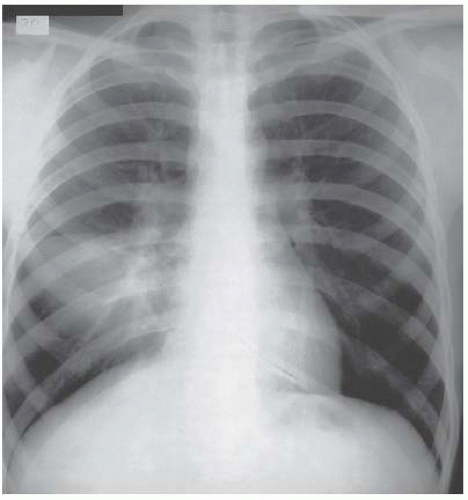
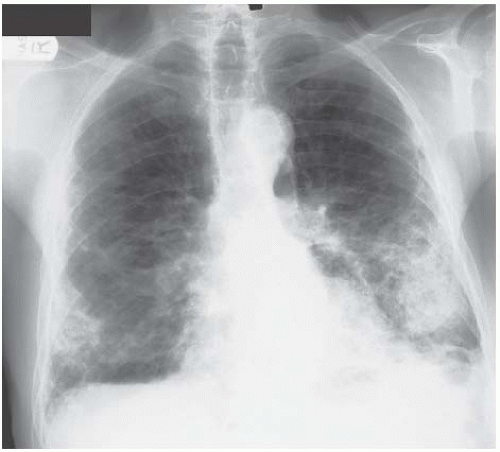
If the shadowing is predominantly peripheral, eosinophilic pneumonia should be considered (Fig. 3.5). In the majority of cases, a circulating eosinophilia provides a clue to the diagnosis but this isn’t always present and lung biopsy may be needed to confirm the nature of the eosinophilic infiltrate.
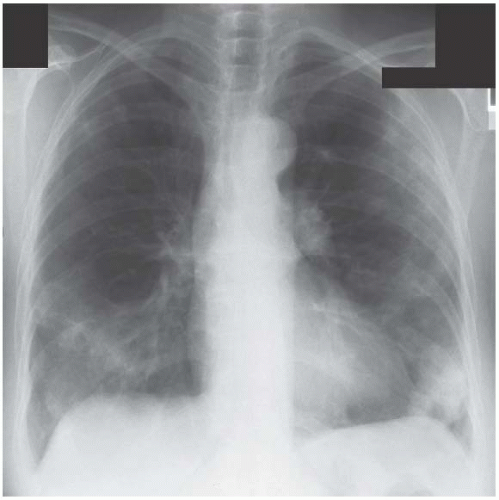
Other non-infective but inflammatory conditions cause multisegmental consolidation. Figure 3.6 is an example of cryptogenic organizing pneumonitis, an inflammatory condition with a clinical presentation that commonly mimics pneumonia.
Both of these conditions require treatment with corticosteroids, emphasizing the management implications of distinguishing non-infective causes of consolidation.
Wegener’s granulomatosis is a vasculitic disease, which characteristically produces multiple areas of consolidation when it affects the lungs. These lesions commonly cavitate (Fig. 3.7).
On rare occasions, sarcoidosis causes multisegmental consolidation (Fig. 3.8). In Fig. 3.9 there are nodules as well as patches of consolidation.
Malignant infiltrates (haematological, lymphoproliferative and solid tissue tumours) are also in the differential diagnosis of multisegmental consolidation.
Consider non-infective pathology if there are multiple areas of consolidation. A thorough interpretation of the radiograph will raise your suspicions and help to ensure early appropriate treatment. Even though the story may sound like infection, this radiographic pattern can also be caused by disease processes that will not respond to antibiotics.
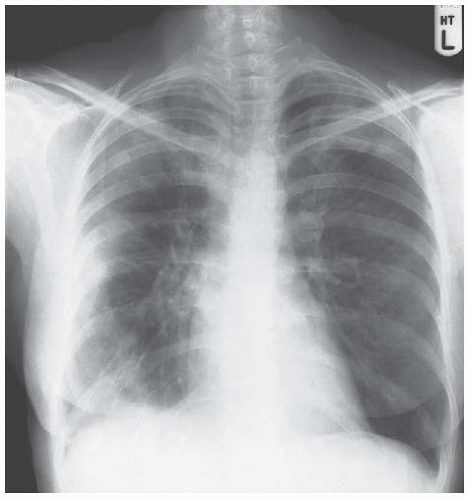 Figure 3.5 Eosinophilic pneumonia. The peripheral consolidation in this example is asymmetric with involvement of all areas of the right lung but only the apex on the left. |
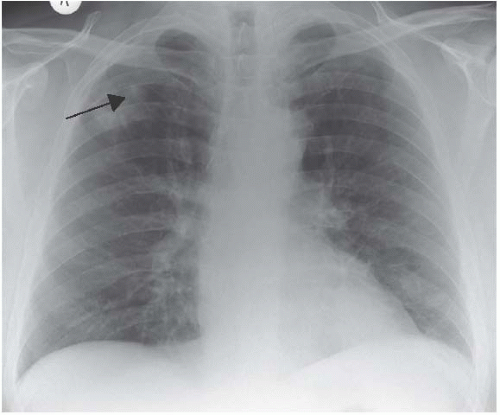 Figure 3.7 Wegener’s granulomatosis. There are patches of consolidation in the right upper and left lower zones and cavitation can be seen in the former. |
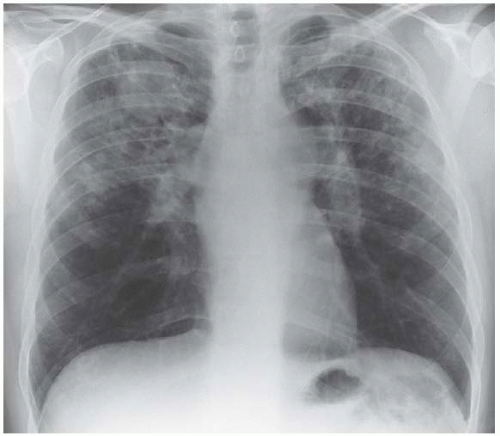 Figure 3.9 Nodules are present as well as confluent areas of consolidation. The bilateral hilar lymphadenopathy is a clue to the diagnosis of sarcoidosis. |
Other recognizable radiographic patterns include:
Pulmonary oedema. This has a characteristic perihilar distribution and the epithet, ‘Bat’s wing of death’, though unfortunate, is often appropriate morphologically (Fig. 3.10).
Aspiration pneumonia is one cause of bilateral consolidation in the lower zones. Loss of volume may accompany the consolidation as a result of bronchial obstruction from aspirated.
Alveolar haemorrhage is commonly perihilar in distribution but this isn’t totally reliable – the major haemorrhage shown in Fig. 3.11 has resulted in extensive
bilateral consolidation. There is no air-bronchogram within the shadowing because blood is filling the airways as well as the alveoli.
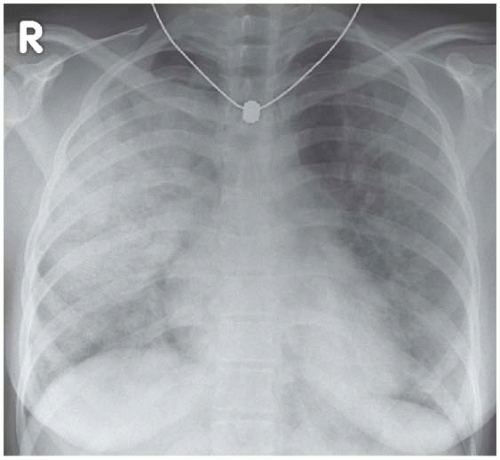 Figure 3.11 Extensive intrapulmonary haemorrhage in a patient with idiopathic pulmonary haemosiderosis. |
In contrast:
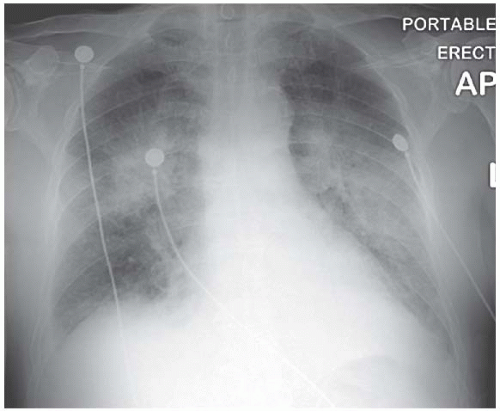
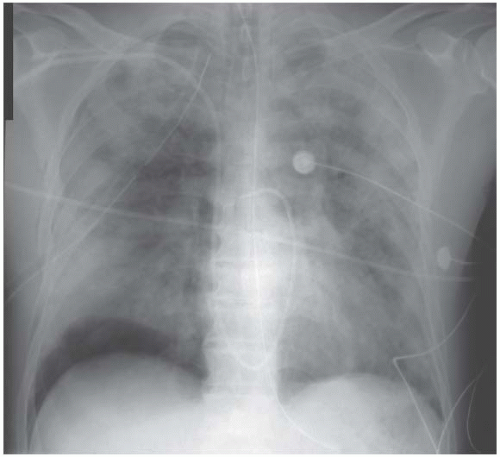
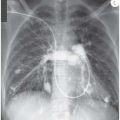
There are no distinguishing radiographic features displayed by the bilateral alveolar filling pattern in the example of pulmonary alveolar proteinosis shown in Fig. 3.12 and the same applies to most cases of Pneumocystis carinii pneumonia (Fig. 3.13) and alveolar cell carcinoma (Fig. 3.14). The consolidation in Fig. 3.15 is as a result of the adult respiratory distress syndrome and, although the multitude of tubes and wires may be a clue as to aetiology, there is nothing diagnostic about the radiograph per se.
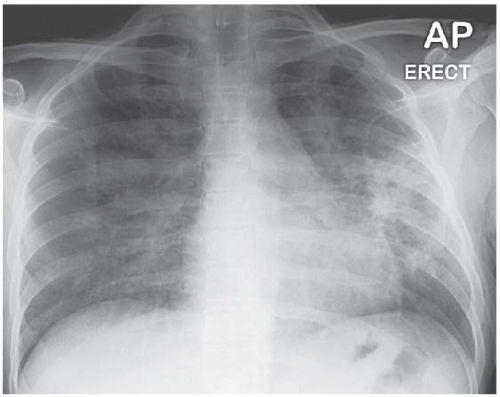 Figure 3.13 Extensive bilateral alveolar-filling pattern in Pneumocystis carinii pneumonia complicating human immunodeficiency virus (HIV) infection. |
The last four cases illustrate the fact that lungs become consolidated in a variety of pathological conditions and that the radiographic appearance of ‘alveolar filling’ is ubiquitous and non-discriminatory in many instances.
What is the density of the shadowing?
Sometimes consolidation is of low density, but more commonly it is medium dense, representing as it does alveoli filled with fluid, cells, infective organisms or a mixture of these components. Heavy-density shadowing is not seen except under unusual circumstances, for example if a radiodense foreign body is responsible for bronchial obstruction (see Clinical connections).
A middle-aged man with a heavy alcohol intake presented one weekend septic with pneumonia. He had consolidation with no air bronchogram in the right middle and lower lobes and there appeared to be a calcified area approximately 1 cm2 in the right mid-zone. At bronchoscopy, I retrieved a vertebral body of a small mammal (presumably a rabbit) from the intermediate bronchus. He recovered remarkably well despite the fact that Actinomyces was present in bronchial aspirate. Although he had no recollection of eating rabbit or anything similar, this was presumably aspiration pneumonia.
Cavitation within an area of consolidation indicates a particular infecting organism (Staphylococcus, Mycobacteria and Gram-negative organisms are in the differential diagnosis [Figs 3.16 and 3.17




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree