Abstract
Objective
Mouse models of hindlimb ischemia (HLI) are used to study peripheral arterial disease and evaluate novel therapies. Contrast-enhanced ultrasound (CEUS) is a noninvasive perfusion measurement technique that is increasingly being employed in these models. The objective of this study was to evaluate two models of severe HLI by CEUS to characterize perfusion recovery and muscle perfusion patterns.
Methods
Mice undergoing double femoral artery ligation were measured by CEUS and laser Doppler perfusion imaging (LDPI) at baseline and 1–150 d postsurgery. A second group undergoing femoral artery ligation and excision was measured 1–28 d postsurgery.
Results
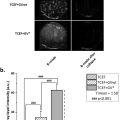
By LDPI, both surgeries showed robust perfusion recovery by 14 d postsurgery. However, by CEUS only a ∼40% perfusion recovery plateau was reached in either group. These results are consistent with our previous work, employing a less severe single femoral artery ligation, that showed perfusion in the ischemic limb does not return to normal by 150 d postsurgery. Cluster analysis of muscle perfusion patterns indicated 3–5 different patterns at day 1 postsurgery. The double ligation model yielded significantly less variable perfusion patterns, suggesting that it can provide more reproducible results.
Conclusion
Contrary to LDPI, perfusion as measured by CEUS never fully recovers after hindlimb surgery, even when followed 28–150 d postsurgery. Individual mice can manifest different patterns of muscle perfusion to the same surgery, but these patterns are conserved within and between different surgical techniques. These results may have significant implications for the evaluation of novel therapeutics to treat PAD in mice.
Introduction
Peripheral arterial disease (PAD) is the lack of perfusion to the limbs due to obstructive atherosclerosis [ ]. PAD has various presentations including asymptomatic, intermittent claudication, and the most severe form of the disease, critical limb ischemia, where gangrene may be present necessitating amputation [ , ]. Very few therapies exist for PAD, and mouse models of hindlimb ischemia (HLI) play important roles in providing mechanistic insight into disease pathology and in evaluating novel therapeutic approaches for potential clinical translation [ ].
Mouse models of PAD are most often HLI models where unilateral femoral artery ligation(s) and/or an excision of the femoral artery is performed [ ]. Most commonly, laser Doppler perfusion imaging (LDPI) is used to measure perfusion recovery over time. However, we have recently shown that LDPI does not correlate with fluorescent microsphere, histological, or photoacoustic microscopy measurements of perfusion late after HLI [ ]. We demonstrated contrast-enhanced ultrasound (CEUS) does correlate well with these methods in an HLI model employing a single femoral ligation. This notwithstanding, it is unknown how the results of CEUS and LDPI analysis might compare in more severe HLI models that seek to emulate more advanced forms of PAD.
Another advantage of using CEUS is that perfusion can be visualized in individual muscle groups. Spatial information about perfusion is lacking in the literature, as the techniques that can provide spatial information are typically destructive or limited to anatomical vascular structure rather than functional perfusion information. For example, micro-CT and angiography can provide good anatomic structure, but limited functional perfusion information [ ]. Other imaging techniques such as MRI or PET can provide spatial and perfusion information, but are much more costly and can suffer from poor resolution in small animals with low flow rates [ , ]. Compared to ultrasound, these techniques often require more resources including a dedicated support staff.
Here, we evaluate perfusion recovery in two mouse models of severe HLI using both CEUS and LDPI. We also evaluate muscle perfusion patterns after surgery in the three most common HLI models and apply k -means clustering to explore variation within and between surgical techniques.
Materials and methods
Mice
Male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were provided with water and standard chow ad libitum . Mice were housed 1–4 animals per cage and in a room with a 12 h light cycle. Cages had standard corn cob bedding except postsurgery when Iso-pad bedding (Envigo, Indianapolis, IN, USA) was used until the mice were ambulating normally. The Institutional Animal Care and Use Committee of the University of Virginia approved all animal experiments.
Mouse models of HLI
Mice (10–12 wk old) were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). The left medial leg was shaved and depilated and the area was prepared for aseptic surgery. A heating lamp was used to maintain body temperature during surgery. An incision was made over the femoral artery and the following procedures for each type of surgery were followed:
Single ligation- 6-0 silk sutures were used to ligate the femoral artery proximal to the lateral circumflex femoral artery [ ].
Double ligation- 6-0 silk sutures were used to ligate the femoral artery proximal to the iliacofemoral artery and the popliteal/saphenous bifurcation [ ].
Ligation/excision- 6-0 silk sutures were used to ligate the femoral artery proximal to the pudendoepigastric trunk and the popliteal/saphenous bifurcation [ , ]. The segment between the sutures was then excised. Care was taken to not damage nearby structures such as the femoral nerve and vein. Buprenorphine-SR (0.5 mg/kg) was administered subcutaneously after surgery and re-administered as needed every 48 h or as recommended by veterinary staff based on facial grimace score and burrowing behavior. Mice were euthanized and excluded if necrosis reached the heel.
Microbubble preparation
Microbubbles were prepared as previously described [ , ]. Briefly, microbubbles were formed by dispersion in saline and have a decafluorobutane gas core and a lipid monolayer shell composed of 1,2-distearoyl- sn -glycero-3-phosphocholine and PEG stearate. The presence of any microbubbles larger than 7 μm was minimized by flotation at normal gravity. The concentration of microbubble stocks was measured approximately every 2 weeks (Beckman Coulter, Indianapolis, Indiana, USA). Microbubbles were diluted with sterile saline directly before administration to a concentration of 1 × 10 9 MBs/mL and infused at 10 μL/min.
Contrast-enhanced ultrasound
Imaging was performed on an Acuson Sequoia C512 system with a 15L8W transducer (Siemens, Munich, Germany) as previously described [ ]. Briefly, mice were anesthetized with 1%–2% isoflurane in air, placed prone on a heated stage with legs extended, and the feet were secured. Mice were anesthetized with isoflurane in air rather than oxygen because the use of air as the carrier gas limits the circulation lifetime of MB contrast agents [ ]. A 27G catheter filled with heparinized saline (100 units/mL) was inserted in the tail vein and secured. Ultrasound gel was put on the calves and the transducer was positioned perpendicular to the calves. Previously optimized scanning parameters were used (frequency: 7 MHz, dynamic range: 100 dB, gain: −10, imaging mechanical index: 0.2, burst mechanical index: 1.9, and burst time: 1 s) [ ]. Video of the ultrasound imaging was captured in real-time. The microbubble solution (1 × 10 7 MB/min) was infused via tail vein catheter. After reaching steady-state, burst pulses were applied once per minute for 10–15 min for data acquisition. The burst pulses destroy only the microbubbles within the field of view, while microbubbles from upstream continue to flow into the scan plane thus enabling perfusion to be quantified [ ]. CEUS measurements were always taken after LDPI measurements because it has previously been reported that ultrasound-mediated microbubble cavitation can transiently increase hindlimb perfusion in mice for up to 24 h [ ]. However, we did not observe this phenomenon during our initial test/retest study of CEUS performed at 3–4 d intervals over the course of 11 d [ ].
A custom MATLAB (MathWorks, Natick, Massachusetts, USA) script was used for analysis as previously described [ ]. The image intensity over time of each flash-replenishment sequence was fit to y = A × (1 − e − βt ) where A represents blood volume, beta represents blood velocity, and A × β represents blood flow [ ]. An average ischemic/control hindlimb A × β ratio was used as the CEUS output metric.
Perfusion pattern analysis
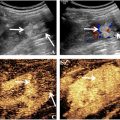
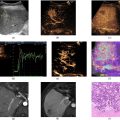
CEUS imaging data from day 1 postsurgery was used for perfusion pattern analysis. A total of 46 mice (17 single ligation, 18 double ligation, and 11 excision/ligation) were analyzed. Raw data for the single ligation surgery was from a previous study [ ], however, a perfusion pattern analysis was not performed at that time. These data were retrospectively analyzed and included in this analysis for comparison with the other two models. A B-mode image, a contrast mode image before microbubble infusion (background), and a contrast mode image during steady-state microbubble infusion were extracted from the video data for each mouse. Data were analyzed using a custom MATLAB (MathWorks, Natick, Massachusetts, USA) script. The background image was subtracted from the contrast image to ensure any artifacts were removed before quantification. The B-mode image was used to determine the region of interest in the same way as was done with the video data. The region of interest was further broken into four major muscle groups plus the skin based on published microscopy images of the mouse hindlimb ( Figs. 1 and 2 ) [ ]. This segmentation scheme grouped some of the smaller muscles with larger ones because the small muscles are difficult to correctly segment and contribute minimally to the total signal. The average image intensity in each major muscle region was then quantified. k -means clustering was performed on these muscle region intensities for two to 10 clusters [ , ]. The number of clusters was optimized using silhouette analysis [ ].


Laser doppler perfusion imaging
LDPI was performed as previously described using the PeriCam PSI (Perimed, Sweden) [ ]. Mice were anesthetized with 1%–2% isoflurane in oxygen, and calves were shaved and depilated. Mice were placed prone and the feet were secured in place. Three to five measurements were taken and data were analyzed using the included PimSoft analysis software. Regions of interest were selected around the feet and the image intensity was averaged over all measurements. A left/right (ischemic/control) foot ratio was calculated.
Histological analysis
Mice were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). Mice were then perfused through the left ventricle with heparinized saline (10 units/mL) followed by 4% paraformaldehyde. Skeletal muscles of the calf were harvested and incubated in 4% paraformaldehyde at 4 °C for 48–72 h. The tissue was then rinsed three times in saline and placed in 15% sucrose overnight followed by 30% sucrose overnight. The resulting tissue was flash-frozen in OCT and 10 μm sections were cut and stained with H&E.
Statistical analysis
Power analysis for sample size was performed using standard deviation values from our previous study that employed a similar experimental design [ ]. Animals were randomly assigned to experimental groups. All results are expressed as the mean ± standard error of the mean. A linear mixed model with Bonferroni correction was used to analyze the longitudinal LDPI and CEUS data using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). A p value <0.05 was considered significant.
Results
Surgical mortality and exclusions
A total of 32 mice were enrolled in these studies, but 3 mice succumbed to complications of surgery prior to imaging on day 1 and were therefore excluded from data analysis. Mortality due to surgery was thus <10% and all mice that survived for 24 h postsurgery successfully completed their assigned imaging studies. However, we were unable to analyze images from two mice at day 1 after double-ligation surgery due to file corruption, and 8 of the 18 doubly-ligated mice in the 0–14 d study were part of an extended study that lasted 150 d. Tables summarizing the number of mice analyzed per time point and associated degrees of freedom for each study are appended as Supplementary Data .
Double ligation surgery results in sustained perfusion deficit
To evaluate perfusion recovery over time in the double ligation HLI model, mice were imaged at baseline and days 1, 4, 7, 14, 28, 60, 90, and 150 after surgery by LDPI and CEUS ( Fig. 3 ). By LDPI, perfusion in the ischemic limb was reduced to 35% ± 3% of the control limb day 1 postsurgery. Perfusion in the ischemic limb then increased approximately linearly until day 14 postsurgery, at which time it returned to presurgery levels and remained steady through day 150 postsurgery. By CEUS, perfusion in the ischemic limb was reduced to 6% ± 2% of the control limb on day 1 postsurgery. In contrast to LDPI, perfusion by CEUS in the ischemic limb modestly peaked at ∼52% on days 7 and 14 postsurgery and then plateaued at ∼35% of the control limb through the end of the study. Measurements by LDPI and CEUS are significantly different at all time points ( p < 0.005, n = 18 for baseline, n = 16 for 1 d, n = 18 for 4 to 14 d, and n = 8 for 28–150 d time points).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree