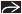CT in Head & Neck Cancer
Michelle A. Michel, MD
Key Facts
Clinical Implications
CECT: Best initial modality for patient presenting with neck mass of uncertain etiology
Fast; good-quality imaging is readily reproducible
Less affected by breathing & swallowing artifacts than MR in infrahyoid neck/mediastinum
Superior for detecting cortical bone erosion & intratumoral calcification
Helpful for determining tumor extent & size, identifying nodal disease, assessing response to therapy, & restaging
CT also useful for image-guided biopsy
Preferred imaging modality for tumors of oropharynx, hypopharynx, & larynx
Tumor volume measurements with CT correlate with local control & outcome for supraglottic, glottic, pyriform sinus, & nasopharyngeal carcinoma
Imaging Approaches
MDCT: Scan from sella to thoracic inlet or to carina
Coronal & sagittal reconstructions aid in determining tumor extent
Special Techniques/Dynamic Maneuvers
“Puffed cheek”
Modified Valsalva
Phonation
Open mouth
3D endoscopic view
Risk Factors & Complications
Contrast reactions are uncommon, particularly with use of nonionic, low osmolality agents
Patients with renal insufficiency, dehydration, & paraproteinemias are at increased risk for contrastinduced nephropathy (CIN)
TERMINOLOGY
Definitions
• CT: Fundamental cross-sectional imaging modality for evaluating H&N cancer
CLINICAL IMPLICATIONS
Clinical Importance
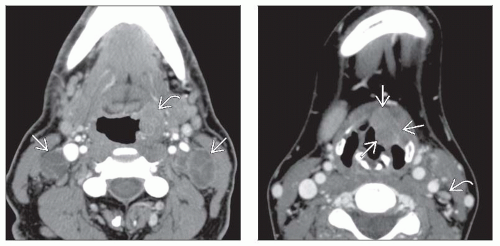
CECT is best initial modality for patient presenting with neck mass of uncertain etiology
CECT is excellent modality for SCCa staging
Determining tumor extent & size, identifying nodal disease, assessing response to therapy, & evaluating for recurrent disease
Preferred by many for determination of pathologic lymph nodes
CT is superior for detecting cortical bone erosion & intratumoral calcification
Preferred imaging modality for tumors of oropharynx, hypopharynx, & larynx
Fast & less affected by breathing & swallowing artifacts in infrahyoid neck/mediastinum
Better detail of parathyroid glands than MR (less affected by breathing & pulsation artifacts)
Good quality imaging readily reproducible
IMAGING APPROACHES
Multidetector Computed Tomography (MDCT) with Multiplanar Reformations
CECT with coverage from skull base to thoracic inlet required for SCCa staging; to evaluate left recurrent laryngeal nerve course, coverage must extend through carina
High-quality, thin-slice images reconstructed in multiple planes from single data set
Permits high-resolution images unaffected by movement
Decreased scan time allows for reduced contrast dose
Minimizes radiation exposure
Bismuth shields may be used to ↓ thyroid & lens exposure
Slight neck extension allows exclusion of orbits
NECT with bone algorithm may be performed after MR in nasopharyngeal & oral cavity tumors to identify cortical bone invasion
Reformatted Images
Sagittal & coronal reformations are very helpful for assessing craniocaudal extent of hypopharyngeal, laryngeal, & tracheal lesions
Allows 3rd plane measurement of mass or nodes
Coronal
Identifies skull base & orbital invasion
Assesses transglottic extension of laryngeal tumors
Sagittal
Best identifies invasion of preepiglottic fat by tongue base & supraglottic tumors
IMAGING PROTOCOLS
Neck
Craniocaudal helically acquired sections obtained from skull base (sella) through carina
Coverage through carina necessary for evaluation of left true vocal cord (TVC) dysfunction
Slice thickness ˜ 0.6-1.25 mm
Head positioned with beam parallel to inferior orbitalmeatal line with no gantry tilt
Additional scans angled above maxillary and below mandibular dentition to exclude amalgam artifact
Images reconstructed with standard (soft tissue) algorithm & edge-enhancing (bone) algorithm
Soft tissue = ww 350/wl 40; 2.5 mm
Bone = ww 3,000/wl 800; 0.625-1.25 mm
Other parameters: kVp = 120; mA range = 100-800; gantry rotation time ˜ 0.7 sec (↑ for larger patients); beam collimation = 20 mm; detector configuration = 32 × 0.625; pitch ˜ 1; table speed ˜ 20 mm/rotation
Perform with patients in quiet respiration, not holding breath
Contrast
Standard recommended dose = 90-120 mL
Standard dose administered if GFR > 60
Tissue enhancement affected by body weight (weight-adjusted dose recommended for higher body weights)
< 80 mL contrast (1.3 mL/kg with iodine concentration of 300 mg/mL) results in suboptimal vessel contrast
Contrast rate ˜ 2 cc/sec after ˜ 60 second scan delay
Split bolus technique for contrast administration may improve lesion/vascular enhancement
Saline chaser technique (40 mL saline added on top of contrast in injector)
Clears IV catheter of contrast & keeps tighter contrast bolus
Avoids pooled contrast in arm veins, reducing perivenous artifacts
May not show any difference in neck vessel attenuation (arterial vs. venous)
Side of injection may have little effect on mean attenuation value in vessels
Sinonasal
Thin-slice axial data set acquired with MDCT and reconstructed in coronal ± sagittal planes
Coronal reformations performed perpendicular to hard palate, extending from nasal vestibule through sella
Reformat thickness: 1.0-1.5 mm
Sagittal reformations performed perpendicular to hard palate
Typically performed without contrast unless there is contraindication to gadolinium-enhanced MR for precise tumor mapping
Specific Techniques/Dynamic Maneuvers
“Puffed cheek”
Improves visualization of oral cavity mucosal tumors along the typically apposed surfaces (gingival & buccal mucosa/oral tongue & gingivae)
Cheeks, gingivae, lips, buccal vestibule, buccinator muscle, pterygomandibular raphe, and retromolar trigone better delineated
Patient blows uniformly through pursed lips
1 mm thick scans performed from hard palate to inferior edge of mandible
Optimized by moving tongue away from hard palate & teeth
Modified Valsalva
Used to improve evaluation of location & extent of hypopharyngeal tumor due to apposition of mucosal surfaces or nasopharyngeal lesions when pharyngeal recesses (fossa of Rosenmüller) are collapsed
Opens the glottis & distends the laryngeal vestibule & pyriform sinuses; improves separation of postcricoid & postarytenoid soft tissues from posterior pharyngeal wall
True & false cords are abducted and poorly depicted
Patient breathes out against resistance of pursed lips (for hypopharynx) or a pursed nose (for nasopharynx)
1 mm thick scans performed from hyoid bone to trachea
Patient training improves success of maneuver
Phonation
Used when true & false vocal cords are not clearly depicted (apposed) when scan performed during apnea or quiet respiration
True & false cords and laryngeal ventricles better depicted
Improves accuracy of determining supraglottic vs. glottic tumors
Patient says “eeeeeeeee” for 10 seconds
1 mm thick scans performed from hyoid bone to trachea
Patient training improves success of maneuver
Open mouth
Used to improve visibility of oral cavity & oropharynx masses obscured by dental amalgam artifact
Improves visualization of soft palate, cheeks, gingivae, mobile tongue
Avoids “missing” areas and double irradiation of areas in the common procedure of tilting scanner gantry to avoid amalgam
Patient opens mouth, and device (e.g., 50 mL syringe) is placed between teeth for stabilization
1-3 mm thick scans obtained from maxilla to mandible in quiet respiration
3D endoscopic view
“Virtual endoscopy”: High-resolution volumetric images processed to provide 3D rendering of mucosal surfaces
Evaluates airway narrowing beyond a stricture not accessible via endoscopy
Most helpful for evaluation of laryngotracheal stenosis
Noninvasive, typically does not require sedation or IV contrast
Evaluation of pediatric subglottic stenosis post intubation
3D reconstructions processed using surface- or volume-rendered techniques
Best for evaluating extent & shape of subglottic & tracheal stenosis
May overestimate stenosis at level of glottis due to adduction of vocal cords
CLINICAL INDICATIONS & UTILITY BY REGION
Role of CT in H&N Cancer Patient
Initial staging
Evaluate size & extent of primary lesion
Tumor volume measurements correlate with local control & outcome for supraglottic, glottic, pyriform sinus, & nasopharyngeal carcinoma
CT also helpful for identifying synchronous or metachronous neoplasms
Hypopharyngeal primaries most likely to have 2nd primary (1/3 synchronous with initial SCCa)
Assess for nodal involvement
Identify metastases: Lung apices, bone, thyroid
Image guidance for biopsy
Directs to most accessible & highest yield site for tissue sampling
Treatment planning (type of surgery, radiation fields, ± chemotherapy)
CT helps to determine resectability of T4 lesions
Critical factors that should be sought on imaging
Tracheal & esophageal extension, laryngeal cartilage invasion, preepiglottic fat involvement
Bone invasion: Mandible, maxilla, & skull base
Dural spread, perineural tumor spread, orbital invasion, & brachial plexus invasion
Arterial encasement, prevertebral fascia involvement, mediastinal infiltration
Assess response to therapy & potential complications
Baseline imaging after therapy used to assess for residual tumor & serves as roadmap for future studies
Performed 8-10 weeks post chemoradiation, 10-12 weeks post surgery
Surveillance
Variable frequency of follow-up studies (3- to 6-month intervals)
Compare to baseline examination to detect recurrent disease & re-stage
Recurrence most often in 1st 2 years post treatment