PET is a versatile imaging modality widely used in oncology for diagnosing, staging, predicting outcomes, and surveillance for a variety of cancers. In radiation oncology, combining PET and computed tomography imaging can markedly enhance treatment planning through improved target volume delineation. This review examines data and clinical approaches across 3 major cancer types to evaluate the role of PET in target volume delineation, with data and current approaches for thoracic, genitourinary, and head and neck malignancies detailed. Additionally, it emphasizes various practical applications of PET in radiation therapy planning, several of which have been recently demonstrated in clinical trials.
Key points
- •
PET/computed tomography (CT) combines functional and anatomic imaging to optimize target volume delineation for radiotherapy treatment planning.
- •
PET/CT is being used to guide the delivery of simultaneous integrated boosts and develop mid-treatment adaptive plans.
- •
More work is needed to establish PET/CT maximum standard unit value (SUV max ) kinetics and tumor biomarkers that can inform personalized PET-based adaptive radiotherapy approaches.
| CT | computed tomography |
| F18 | fluorine-18 |
| 18F-FDG | 18F-fluorodeoxyglucose |
| FMISO | fluoromisonidazole |
| GTV | gross tumor volume |
| HPV | human papilloma virus |
| NSCLC | nonsmall cell lung cancer |
| PFS | progression-free survival |
| PSA | prostate-specific antigen |
| PSMA | prostate-specific membrane antigen |
| SBRT | stereotactic body radiation therapy |
| SCC | squamous cell carcinoma |
| SCLC | small cell lung cancer |
Introduction
Accurate target volume delineation is a fundamental cornerstone of radiation oncology, as it allows for treatment planning to maximize the delivery of radiotherapy dose to the target (eg, gross tumor volume) and minimize the irradiation dose to surrounding normal tissues. Such accuracy is more important than ever with the increasing use of advanced radiation modalities like intensity-modulated radiation therapy, stereotactic body radiation therapy (SBRT), and proton therapy that all rely on exact knowledge of the tumor volume location to minimize the risk of marginal failures. Discriminating between tumor and normal tissue using conventional imaging modalities, such as computed tomography (CT) and MR imaging, is largely dependent on morphologic features. In contrast, 18F-fluorodeoxyglucose (18F-FDG) PET/CT combines functional and anatomic imaging to assist with distinguishing tumor from normal tissue. Cancer cells typically consume more glucose than normal tissues due to the Warburg effect. FDG is a glucose analog labeled with a radioactive isotope (ie, 18F), so when tumor cells disproportionately consume 18F-FDG, PET/CT imaging can detect increased signal in tumor compared with most normal tissues. The PET to CT fusion provides anatomic context to the areas of increased FDG avidity to further assist with tumor delineation.
The differential rates of 18F-FDG between tumor and normal cells are impacted by several factors: the burden of metabolically active tumor; the expression of the glucose transporter molecules (glucose transporter type [GLUT] 1, GLUT5); and the activity of glycolytic enzymes. For most tumor types, all 3 of these factors are skewed toward increased 18F-FDG uptake by tumor relative to normal tissues. However, some tumors, such as nonsmall cell lung cancer (NSCLC) and small cell lung cancer (SCLC), are characterized by particularly high expression of GLUT1, which increases the applications of 18F-FDG PET/CT for these tumor histologies.
In addition to assisting with target volume delineation, PET/CT imaging also plays critical and well-established roles in tumor detection and staging, the identification of intratumoral hypoxia, target volume selection for dose escalation, treatment response assessment, and postradiotherapy surveillance, as well as an emerging role assessing for the treatment-related complications. This review highlights both existing and emerging evidence supporting the use of innovative PET/CT imaging approaches to enhance radiotherapy target volume delineation that can facilitate improved patient outcomes.
Thoracic
18F-FDG PET/CT is responsible for many innovations in the management of both NSCLC and SCLC ( Table 1 ). It plays a pivotal role in the workup of lung cancer, as it is sensitive in the detection of regional nodal metastases and distant metastases. In fact, the first oncology-related indication to receive 18F-FDG PET/CT Medicare coverage was the staging of newly diagnosed NSCLC. The ability of 18F-FDG PET/CT to accurately detect regional nodal metastases has not only improved pretreatment staging of NSCLC and SCLC but also supported the discontinuation of elective nodal irradiation for locally advanced NSCLC and limited-stage SCLC. In locally advanced NSCLC, 18F-FDG PET/CT-staging-enabled involved-field radiotherapy can dramatically reduce the dose delivered to normal tissue, leading to fewer high-grade adverse events with involved-field radiotherapy compared with elective nodal irradiation.
| PET/CT Innovation | NSCLC | SCLC |
|---|---|---|
| Improved staging to guide appropriate therapy | ✓ , , | ✓ , |
| Target volume reduction through cessation of elective nodal irradiation | ✓ , | ✓ |
| Dose escalation based on pretreatment PET/CT | ✓ | |
| Targeted dose escalation based on mid-treatment PET/CT response | ✓ |
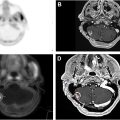
18F-FDG PET/CT imaging can also facilitate improved target volume delineation in scenarios such as radiotherapy for a lung tumor causing atelectasis. In these situations, partial or complete lung collapse causes lung tissue to appear denser, and the boundary between tumor and normal tissue subsequently becomes more challenging to identify. Another common indication for 18F-FDG PET/CT-based treatment planning is reirradiation. Prior lung radiation therapy can lead to dense pulmonary fibrosis, which can conceal the extent of recurrent disease, but functional imaging can help differentiate between tumor recurrence that could benefit from repeat local therapy and less metabolically active adjacent scar tissue.
18F-FDG PET/CT simulation is critical for effective radiotherapy treatment planning and the use of appropriate treatment(s) for patients with NSCLC. It reduces variability in gross tumor volume (GTV) definitions, and it can detect disease progression that has occurred since the time of the initial staging 18F-FDG PET/CT. In NSCLC, when the 18F-FDG PET/CT simulation occurs within 6 weeks of the initial staging 18F-FDG PET/CT, upstaging occurs in 43% of patients: 13% with a change in N stage; 22% with a change in M stage; and 8% with a change in T stage. This upstaging can alter treatment selection and prevent the delivery of inappropriate treatments to NSCLC patients. ,
Local failure is common after concurrent chemoradiation for locally advanced NSCLC, even in the setting of consolidative durvalumab. Local failure may seed new distant metastases, and it is associated with worse overall survival. RTOG 0617 showed that whole-tumor dose escalation—especially using 3-dimensional conformal radiotherapy—for locally advanced NSCLC can be harmful due to increased cardiotoxicity. Therefore, prospective studies have evaluated the use of 18F-FDG PET/CT to identify areas of FDG avidity for radiotherapy dose escalation. In the phase II, multicenter randomized controlled RTEP7–IFCT-1402 trial, patients with inoperable stage III NSCLC without EGFR mutation or ALK rearrangement were randomized to receive either standard chemoradiotherapy (ie, 66 Gy over 33 fractions) or adaptive chemoradiotherapy (up to 74 Gy over 33 fractions). All patients received 2 cycles of induction chemotherapy prior to initiating chemoradiotherapy. Given that the maximum standard unit value (SUV max ) obtained 4 weeks after initiating conventionally fractionated chemoradiotherapy is associated with survival and is potentially actionable, patients in the adaptive arm received a boost only if there was residual uptake on 18F-FDG PET/CT after having received 42 Gy. Most patients in the adaptive arm (71%) received a boost dose, and the adaptive arm demonstrated numerically superior 15-month local control (primary endpoint) compared with the standard chemoradiotherapy arm (77.6% vs 71.2%). Notably, the improved local control with adaptive dose escalation did not come at the cost of increased acute toxicity.
Further support for an adaptive approach comes from a single-arm prospective study of patients with stage II/III NSCLC treated with definitive chemoradiotherapy followed by 18F-FDG PET/CT or CT obtained 1 month after completing chemoradiotherapy. Patients with residual FDG-avid disease within the site of the primary tumor received an SBRT boost (ie, 20 Gy over 2 fractions or 19.5 Gy over 3 fractions) to the primary tumor to achieve ablative dosing. At a median follow-up of 25.2 months, this 18F-FDG PET/CT adaptive approach was associated with good local control (78%) and a slightly elevated grade 3 pneumonitis rate (11.9%), but no grade 4 or 5 toxicities.
Contrary to the findings of RTEP7–IFCT-1402, NRG-RTOG1106/ECOG-ACRIN 6697 recently reported that mid-treatment 18F-FDG PET-adapted radiotherapy dose escalation did not improve 2-year freedom from local-regional progression. However, there are important differences between RTEP7–IFCT-1402 and NRG-RTOG1106/ECOG-ACRIN 6697 worthy of further discussion. First, 29% of patients in the adaptive arm of RTEP7–IFCT-1402 did not have residual uptake on 18F-FDG PET/CT after receiving 42 Gy over 21 fractions and subsequently did not receive a boost, yet all patients in the adaptive arm of NRG-RTOG1106/ECOG-ACRIN 6697 had FDG-avid residual disease after receiving 46.2 Gy over 20 fractions and received a boost. A potential explanation for the incongruent mid-treatment 18F-FDG PET/CT responses is that patients in RTEP7–IFCT-1402 received (nonstandard) 2 cycles of induction chemotherapy prior to initiating definitive chemoradiotherapy whereas patients in NRG-RTOG1106/ECOG-ACRIN 6697 did not receive induction chemotherapy. In NSCLC, chemotherapy suppresses FDG-uptake signal inside tumors. , It is possible that receiving 2 cycles of induction chemotherapy prior to definitive chemoradiotherapy further suppressed FDG uptake signal for a sizable minority of patients whose tumors are particularly sensitive to cytotoxic therapy. Perhaps additional radiotherapy would have been unnecessary and potentially deleterious for this subgroup of patients. A secondary analysis of RTOG 0617 showed that patients with radiosensitive genotypes (based on ERCC1/2 genotypic signature) had superior survival when treated with 60 Gy rather than 74 Gy; conversely, patients with radioresistant genotypes had superior survival with 74 Gy rather than 60 Gy.
Another potential explanation for the incongruent findings is the NRG-RTOG1106/ECOG-ACRIN 6697 trial used a moderately hypofractionated radiotherapy regimen with concurrent chemotherapy. The majority of studies assessing 18F-FDG PET/CT SUV max kinetics during and after (chemo-) radiotherapy as a prognostic biomarker for locally advanced NSCLC were performed using conventionally fractionated (chemo-) radiotherapy rather than moderately hypofractionated chemoradiotherapy. Given that activated immune cells demonstrate elevated metabolic activity, the acute immune response induced by moderately hypofractionated radiotherapy could have been mistaken for residual FDG-avid disease, leading to overtreatment of a fraction of patients and lymphocyte depletion, which is associated with worse disease control and survival in NSCLC.
Local failure is also common in limited-stage SCLC, and the use of a high-dose accelerated hyperfractionated simultaneous integrated boost to target FDG-avid disease based on 18F-FDG PET/CT was recently assessed in a multicenter phase III randomized controlled trial. The study found that using a high-dose accelerated hyperfractionated simultaneous integrated boost (54 Gy over 30 fractions BID) compared with standard-dose hyperfractionated radiotherapy (45 Gy over 30 fractions) was associated with increased overall survival (median: 60.7 vs 39.5 months, P =.003) and similar toxicity. The similar toxicity, despite dose escalation, was attributed to the use of volumetric arc therapy and the omission of elective nodal irradiation.
Genitourinary
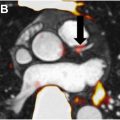
Prostate-specific membrane antigen (PSMA)-PET imaging is revolutionizing radiotherapy planning for men with intermediate-risk to high-risk prostate cancer by offering greater precision in disease staging. By detecting metastases and extra-prostatic spread more accurately than conventional imaging techniques, PSMA-PET allows for a personalized approach to treatment, often resulting in changes to the radiation field or dose. Its combination with multiparametric magnetic resonance imaging has shown promising results in better defining the prostate tumor for targeted radiation therapy. While some studies have demonstrated that PSMA-PET improves tumor volume delineation and treatment planning, the impact on patient outcomes has yet to be defined. Ongoing clinical trials will clarify whether PSMA-PET can outperform traditional imaging approaches, such as CT for the assessment of abdominopelvic lymph nodes and technetium-99m bone scintigraphy for the assessment of bone metastases, but it already increasingly being employed and will likely become a standard tool in managing higher-risk prostate cancer.
In addition to enhanced staging and improved treatment planning for the treatment of newly diagnosed prostate primary tumors, PSMA-PET imaging has significantly enhanced the detection of recurrent prostate cancer for salvage radiotherapy planning, especially in cases of biochemical recurrence where prostate-specific antigen (PSA) levels increase after initial treatment. Traditional imaging often fails to locate the recurrence at low PSA levels, but PSMA-PET offers superior sensitivity, detecting the nidus of prostate cancer recurrence even at PSA levels below 0.5 ng/mL, which is critical given that lower PSA levels prior to salvage radiotherapy are associated with significantly lower rates of disease progression and improved clinical outcomes. This earlier detection allows for more precise radiation targeting, often leading to changes in treatment plans such as expanded target areas or increased radiation therapy doses. Multiple studies report that PSMA-PET frequently alters radiotherapy management in the salvage setting.
Although Ga-PSMA and 18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) are better at detecting early biochemical recurrences after prostatectomy compared with Fluorine-18 (F18) Fluciclovine (ie, Axumin), especially in patients with low PSA levels, F18 Fluciclovine can be more sensitive with the detection of locoregional recurrence due to Ga-PSMA-11 and 18F-DCFPyL accumulating radiotracer in the bladder. F18 Fluciclovine can also help detect disease lacking PSMA expression.
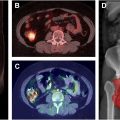
The sensitivity of PSMA-PET/CT imaging has also improved the detection of oligometastatic prostate cancer that is frequently missed with conventional imaging. The impact of PSMA-PET/CT facilitated metastasis-directed therapy was assessed in the oligometastatic phase II randomized ORIOLE trial. That study enrolled patients with recurrent hormone-sensitive prostate cancer and 1 to 3 metastases detectable by conventional imaging who had not received androgen deprivation therapy (ADT) within 6 months of enrollment. Patients were randomized to receive SBRT or observation. Treatment with SBRT improved median progression-free survival (PFS, not reached vs 5.8 months, P =.002). Moreover, total consolidation of all PSMA-avid lesions decreased the risk for new lesions at 6 months. PSMA-PET/CT imaging, such as Ga-68 PSMA-11, is also notable in advanced prostate cancer given that it is used to determine eligibility for Lu-177 PSMA-617 therapy in metastatic castration-resistant prostate cancer patients who have progressed through both taxane-based chemotherapy and an androgen receptor pathway inhibitor. In the recent phase III randomized VISION trial (NCT03511664), treatment with Lu-177 PSMA-617 plus standard-of-care therapy was associated with superior PFS (median, 8.7 vs 3.4 mo, P <.001) and overall survival (median, 15.3 vs 11.3 mo, P <.001) compared with standard-of-care therapy. A more recent phase II randomized trial, ENZA-p (NCT04419402), tested the androgen receptor inhibitor, enzalutamide, versus enzalutamide plus Lu-177 PSMA-617 for the first-line treatment of metastatic castration-resistant prostate cancer in patients with a positive Ga-68 PSMA-PET/CT. Adding Lu-177 PSMA-617 to enzalutamide in the first-line setting was associated with improved PFS and no increased toxicity.
Although FDG PET/CT tends to be less effective for prostate cancer due to decreased glucose metabolism, it can be helpful in more advanced castrate-resistant prostate cancer—especially with tumors that have low or lost PSMA expression demonstrating heterogenous glucose metabolism. The TheraP trial (NCT03392428) enrolled patients with metastatic castration-resistant prostate cancer refractory to docetaxel and randomized them to cabazitaxel versus Lu-177 PSMA-617. That study found that PSMA-PET SUVmean was a better predictor of response to Lu-177 PSMA-617 than cabazitaxel chemotherapy, but high 18F-FDG-PET metabolic tumor volume was associated with lower responses regardless of treatment with cabazitaxel or Lu-177 PSMA-617. Given these findings and early work showing promising tumor control with the combination of Lu-177 PSMA-617 and SBRT for metastatic hormone-sensitive prostate cancer, there may be a role for using 18F-FDG-PET in addition to Ga-68 PSMA-11 when treating patients with Lu-177 PSMA-617 in order to identify tumors with high 18F-FDG-PET metabolic tumor volume that might benefit from treatment intensification with consolidative SBRT after Lu-177 PSMA-617.
Head and neck
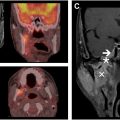
18F-FDG PET/CT is routinely used in head and neck cancer management, particularly for the initial assessment of regional and distant metastases, short-term post-treatment to assess need for neck dissection, and post-treatment monitoring. It has also been studied for its role in target delineation in the primary treatment setting. By incorporating 18F-FDG PET/CT fusion into treatment planning, clinicians can often define GTVs with greater precision compared with using CT or MR imaging alone. , In patients who underwent tumor resections, 18F-FDG PET-based GTVs were found to correspond more closely with actual tumor specimens than other imaging techniques. The sharper GTVs derived from 18F-FDG PET/CT imaging may support safer dose escalation, or de-escalation, although research in this area remains limited. In radiation therapy, additional margins are typically added to GTVs to account for microscopic disease spread and patient setup errors, but the use of more accurate and conformal GTVs through 18F-FDG PET/CT imaging could potentially reduce radiation exposure to surrounding healthy tissues, lowering the risks of toxicities.
18F-FDG PET/CT is especially valuable in cases of head and neck cancers with an unknown primary site, where 18F-FDG PET/CT can identify the primary tumor locations in nearly one-quarter of cases in which CT, MR imaging, and panendoscopy failed to do so. This ability to locate otherwise undetectable primary tumors has significant implications for radiation therapy, as it can prevent unnecessarily large treatment volumes that would otherwise be necessary in cases where a primary tumor cannot be identified, and thus would reduce the likelihood of high-dose irradiation being delivered to areas unnecessarily. ,
Moreover, PET/CT imaging shows promise for use in adaptive therapy—real-time adjustments of the radiation therapy plan to account for anatomic changes during the course of treatment—which can allow for optimal targeting with potential significant sparing of irradiation to normal tissues and is particularly relevant given the rising incidence of radiosensitive cancers like human papillomavirus-positive squamous cell carcinoma (SCC). Tumor hypoxia is associated with worse locoregional and distant control after definitive (chemo-) radiotherapy. 18 F-fluoromisonidazole (FMISO) PET is an effective imaging modality for assessing tumor hypoxia, and hypoxic tumors on FMISO PET are associated with worse locoregional control. Radiotherapy de-escalation efforts in unselected patients with human papilloma virus (HPV)-positive oropharyngeal carcinoma have failed.
However, the recent phase II 30 ROC trial (NCT03323463) used FMISO PET to select HPV-positive oropharyngeal SCC patients for de-escalated cervical lymph node irradiation following surgical removal of their primary tumor but not gross disease in the neck. A baseline FMISO PET was obtained for each of the 158 patients, with hypoxia-negative tumors proceeding with de-escalated cervical lymph node irradiation (30 Gy in 15 fractions) with concurrent platinum-based chemotherapy. Patients with hypoxia-positive tumors at baseline were planned for 70 Gy and 3 cycles of platinum-based chemotherapy; however, 1 to 2 weeks into treatment, a repeat FMISO PET was repeated. If the tumor became hypoxia negative, the patient could finish treatment after receiving only 30 Gy in 15 fractions with 2 cycles of concurrent platinum-based chemotherapy; if the tumor remained hypoxic, the patient was required to complete 70 Gy over 35 fractions with 3 cycles of concurrent platinum-based chemotherapy. Although only 28% of patients had hypoxia-negative tumors at baseline, 78% of patients with hypoxia-positive tumors at baseline became hypoxia negative during treatment; therefore, 84% of HPV-positive oropharyngeal cancer patients in this study were able to be treated with de-escalated chemoradiotherapy, with 2-year rates of locoregional control and overall survival of 95% and 100%, respectively. Notably, there were no late grade 3 to 4 adverse events in the de-escalated chemoradiotherapy arm.
A subsequent multicenter phase II study led by Lee and colleagues enrolled greater than 150 HPV-positive oropharyngeal SCC (T0-2, N1-2c AJCC seventh edition) patients without surgery to the primary site . Nearly 75% of patients were hypoxia-negative and de-escalated to 30 Gy; these patients demonstrated a significant reduction in acute and late toxicities compared with patients receiving 70 Gy. At a median follow-up of 2 years, the 2-year rates of local failure, regional failure, and distant metastasis were 4.2%, 6.9%, and 2.0%, respectively. The 2-year overall survival probability was 99%.
Building off 2 successful phase II trials, this regimen of treatment de-escalation for hypoxia-negative HPV-positive oropharyngeal SCC is now being tested in a phase III multicenter randomized, double-blinded trial (NCT06563479, Fig. 1 ). Eligible patients are required to have newly diagnosed HPV-positive (in terms of both p16 expression and mRNA HPV in situ hybridization) oropharyngeal SCC with or without surgical removal of the primary tumor (AJCC seventh edition cT0-2, N1-2c, M0 on FDG PET/CT). Patients are randomized 2:1 to receive either FMISO PET-personalized chemoradiotherapy ( N = 194) or standard chemoradiotherapy ( N = 97) with the following randomization strata: ≤10 pack years versus greater than 10 pack years smoking, cT0-1 versus cT2, and cN1-N2b versus cN2c (AJCC seventh edition). Standard chemoradiotherapy consists of 70 Gy delivered over 35 fractions with 3 cycles of concurrent cisplatin-based chemotherapy (100 mg/m 2 /cycle). Patients randomized to the personalized chemoradiotherapy initiate conventionally fractionated radiotherapy with cisplatin-based chemotherapy and undergo FMISO PET after 8 to 10 fractions of radiotherapy. Patients with hypoxia-positive tumors are treated with standard chemoradiotherapy (ie, 70 Gy over 35 fractions and 3 cycles of concurrent cisplatin), whereas patients with hypoxia-negative tumors on intratreatment FMISO undergo dose de-escalation and only receive a total of 30 Gy over 15 fractions with 2 cycles of concurrent cisplatin. The primary objective of the study is to demonstrate noninferiority in terms of overall survival for patients with HPV-positive oropharyngeal SCC treated using FMISO PET-based personalized chemoradiotherapy versus standard chemoradiotherapy. Although FMISO PET is not currently FDA-approved, if NCT06563479 demonstrates that hypoxia status on FMISO PET is a predictive biomarker in HPV-positive oropharyngeal SCC in a multicenter phase III randomized trial, this could lead to more widespread testing of FMISO PET for personalized radiotherapy approaches across numerous tumor histologies.