Maxillofacial vascular malformations (MFVMs) are formed due to an error of vascular morphogenesis. They generally grow in proportion to the growth of the affected child but may increase in size secondary to various triggering factors such as increased blood flow, arterial occlusion, and venous thrombosis. The development of an individual lesion, especially if it is high flow, may be stimulated by various factors. High flow in an existing MFVM can induce arteriovenous shunting, which, in turn, increases flow demand, cascading enlargement of the malformation. Increased understanding of these additional physiologic variants may help to define their clinical presentation and evolution and assist in designing therapeutic strategies.
Maxillofacial vascular malformations (MFVMs) are formed due to an error of vascular morphogenesis. They may correspond to a defective remodeling process at the final stages of vessel formation . Although no hereditary MFVMs exist, the defect might be genetically based and secondarily expressed in the first few years of life, such as in Rendu-Osler-Weber syndrome. Vascular malformations generally grow in proportion to the growth of the affected child but may increase in size secondary to various triggering factors such as increased blood flow, arterial occlusion, and venous thrombosis. The development of an individual lesion, especially if it is high flow, may be stimulated by various factors, including endocrine factors (puberty, pregnancy), trauma, iatrogenic insults such as incomplete surgery and proximal embolization, and infection. High flow in an existing MFVM can induce arteriovenous shunting, which, in turn, increases flow demand, cascading enlargement of the malformation. Increased understanding of these additional physiologic variants may help to define their clinical presentation and evolution and assist in designing therapeutic strategies.
Classification and embryogenesis
Vascular malformations are traditionally classified according to the channel abnormalities present and the flow characteristics . The various forms of vascular malformations have been considered by some investigators to be caused by arrests occurring at different stages of vascular development. The rarity of fetal diagnosis of vascular malformations except for the lymphatic malformations suggests an embryonic or fetal cellular defect rather than an already abnormal architecturally demonstrable abnormality.
The development of the head and neck area involves complex changes (rotations, invaginations, migrations) of the tissues during the first weeks in the uterus. During normal development of the head and neck, the vasculature undergoes a series of changes in its branching patterns. Regressions and annexations of arteries account for the unique bidirectional flow in every branch of the head and neck area.
The folds between adjacent buds represent critical areas for capillary maturation, primarily on the venous side. After this has occurred, the arterial system can establish the necessary hemodynamic balance. Delay in bud fusion produces specific arterial anatomic variation. If the maturation of the capillary network is simultaneously delayed, vascular lesions may be seen in association with the arterial variations.
Even if a malformation is present during the development stages, it will remain as a quiescent defect that will be triggered to produce an irreversible fetal, neonatal, child, or adult vascular malformation. Hereditary hemorrhagic telangiectasia produces de novo vascular anomalies over time, often referred to as malformations. The disease gene is recognized to be that for endoglin on chromosome 9q33-34 in some families and activin receptor-like kinase 1 (ACVRL 1) in other families . Families without defects in these two genes have also been reported . The role of growth factors involved in vascular remodeling is probably not restricted to their primary effect; rather, they may serve as multipurpose agents with, for example, a qualitative and quantitative impact on the endothelium (eg, promoters, inhibitors, angiogenetic factors, matrix regulators).
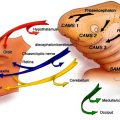
Couly and colleagues demonstrated that endothelial cells of the cephalic region have a regionalized origin from the paraxial mesoderm, which provides blood vessels to specific regions of the face and brain. In general, the neural crest and mesodermal cells originating from a given transverse level occupy the same facial territories, and the two cell types cooperate in myogenesis and in vasculogenesis. Related to this contribution, one can recognize some of the clinical syndromes described in the literature and can postulate a link between apparently unrelated territories. For example, Sturge-Weber syndrome would correspond to a disorder of the rostral mesoderm (medial and lateral) which supplies the prosencephalon and the nasofrontal and maxillary areas. These disorders are collectively called cerebrofacial arteriovenous metameric syndromes .
Diagnosis
The diagnosis of an MFVM is usually made based on clinical history and physical examination. A correct history and careful attention to the child’s complaints with and without his or her parents present are often sufficient to establish the diagnosis. If the cosmetic aspect is dominant, direct communication should always be established with the child to temper the demands of the parents.
Cross section noninvasive imaging such as CT or MR imaging is helpful for assessment of the extent of the disease, associated lesions, or multifocality of involvement. MR imaging is the most useful single imaging modality in the investigation of vascular malformations ( Fig. 1 ). The combination of multiplanar spin echo imaging and flow-sensitive sequences permits characterization of the nature and extent of most lesions. CT is less helpful in defining flow characteristics and the extent of vascular malformations but is useful in demonstrating the nature and extent of bony involvement and the presence of phleboliths, which are pathognomonic of venous malformations. Ultrasound, including Doppler techniques, is a modality for determining tissue and flow characteristics in superficial lesions but is suboptimal in demonstrating the extent of lesions. Plain radiographs are useful in selected patients, mainly to document bony changes. For example, a simple panoramic (Panorex) radiograph may suffice to follow the bony changes in dental arteriovenous malformations. Angiography is reserved for patients in whom a decision has been made to intervene and is generally performed at the same time as embolization. Exceptionally, angiography may be necessary to confirm the diagnosis and to demonstrate the extent of the soft tissue capillary or arteriovenous malformations or fistulas. The advent of three-dimensional rotational angiography is expanding our ability to study intraosseous arteriovenous malformations.
Diagnosis
The diagnosis of an MFVM is usually made based on clinical history and physical examination. A correct history and careful attention to the child’s complaints with and without his or her parents present are often sufficient to establish the diagnosis. If the cosmetic aspect is dominant, direct communication should always be established with the child to temper the demands of the parents.
Cross section noninvasive imaging such as CT or MR imaging is helpful for assessment of the extent of the disease, associated lesions, or multifocality of involvement. MR imaging is the most useful single imaging modality in the investigation of vascular malformations ( Fig. 1 ). The combination of multiplanar spin echo imaging and flow-sensitive sequences permits characterization of the nature and extent of most lesions. CT is less helpful in defining flow characteristics and the extent of vascular malformations but is useful in demonstrating the nature and extent of bony involvement and the presence of phleboliths, which are pathognomonic of venous malformations. Ultrasound, including Doppler techniques, is a modality for determining tissue and flow characteristics in superficial lesions but is suboptimal in demonstrating the extent of lesions. Plain radiographs are useful in selected patients, mainly to document bony changes. For example, a simple panoramic (Panorex) radiograph may suffice to follow the bony changes in dental arteriovenous malformations. Angiography is reserved for patients in whom a decision has been made to intervene and is generally performed at the same time as embolization. Exceptionally, angiography may be necessary to confirm the diagnosis and to demonstrate the extent of the soft tissue capillary or arteriovenous malformations or fistulas. The advent of three-dimensional rotational angiography is expanding our ability to study intraosseous arteriovenous malformations.
Treatment goal and strategy
MFVMs are challenging to treat and require the skills of multiple disciplines. Management of these lesions is best achieved by a specialist who understands the various clinical expressions of the problem, the natural history of the lesion, and the needs of the child. Such a specialist diagnoses the lesion, establishes clinical and morphologic objectives, and then introduces the problem to other specialists for management strategies. Over the last 10 years, the advancement of surgical techniques aided by preoperative, intraoperative, or postoperative embolization has created a new group of specialists combining competence in endovascular, plastic and reconstructive head and neck, and maxillofacial surgery in children .
Because they are usually nonlethal lesions, the primary goal of treatment is to restore and preserve function, stop and control bleeding, and improve or restore cosmesis. Partial treatment in a less risky and less invasive method may be more beneficial to the patient than aggressive curative treatment. MFVMs in children under 10 years of age may interfere with natural growth and maturation of the maxillomandibular frame, causing malocclusion of the mouth or modeling defects owing to external pressure on the forming bones or sinuses. Early intervention can arrest or even reverse such changes. The main indications for early management of vascular malformations are as follows:
Dental arcade stabilization
Osseous remodeling
Recurrent hemorrhagic complications
Mass effect (swallowing, growing)
Episodic swelling and airway compromise
Neurologic impairment
Several questions should be answered to set up an appropriate treatment goal for a child with an MFVM: (1) the nature of the malformation, (2) the type and extent of existing damage, (3) the potential future development of the lesion, (4) the ability to arrest the progression of the disease and to restore damaged function, and (5) the future availability of newly developing treatment techniques.
Specific problems and treatment in each subcategory of MFVM are discussed in the following sections.
Arteriovenous Shunts
An arteriovenous malformation consists of a nidus or network of abnormal vascular channels with feeding arteries and draining veins. Except for extremely rare high-flow lesions, which may present with cardiac overload in neonates and infants, most soft tissue arteriovenous malformations are asymptomatic in the first 1 to 2 decades of life. They often manifest as a cutaneous blush with or without underlying soft tissue hypertrophy. Clinical findings include local hyperthermia, prominent pulsations, thrill, and bruit. Development of these lesions often seems to be precipitated by hormonal factors (puberty, pregnancy, and hormone therapy), trauma, infection, or iatrogenic causes (surgery, embolization). Close follow-up is essential for an arteriovenous malformation because it may extend, especially after incomplete surgical intervention or proximal embolization, into surrounding tissues or territories that initially did not appear to be involved. Venous hypertension results in tissue ischemia, ultimately leading to pain and skin ulceration, often associated with severe bleeding.
If complete eradication cannot be obtained with combined approaches for symptomatic arteriovenous malformations, the authors recommend partial, targeted endovascular embolization with a liquid agent to control the lesion. Surgical partial treatment often triggers expansion of the arteriovenous lesions and should be avoided unless life-threatening bleeding cannot be controlled by transarterial or direct percutaneous embolization. In many cases, the subsequent increase of the abnormal network of vessels after partial surgical resection of the lesion is difficult to treat because it involves normal reactive vascularization. Any attempt at this stage to improve the appearance may lead to tissue ischemia. The authors also try to delay major facial surgical reconstructions involving the skeleton before the completion of maxillofacial growth.
Soft tissue arteriovenous malformations
Arteriovenous malformations can involve the soft tissues in various extensions.
Intramuscular arteriovenous malformations
Intramuscular arteriovenous malformations may be associated with pain (eg, trismus). These arteriovenous lesions are rarely strictly limited to a single muscle; when they are, they usually involve a masticator muscle. They are seldom of the arteriovenous type but are rather venous lesions. Some lesions are small and clinically difficult to demonstrate; they appear as recurrent hematomas, particularly in the masseteric muscle where lysed hematoma may be diagnosed as a cystic lesion. Surgical exploration of these lesions demonstrates small malformations on the wall of the cavity.
Cutaneous arteriovenous malformations
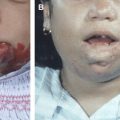
Cutaneous arteriovenous malformations initially demonstrate a superficial blush discoloration and warmth ( Fig. 2 ). As they develop, the color intensifies and tortuous, tense veins appear. Dystrophic changes, ulceration, bleeding, and persistent pain may follow. MR imaging confirms the diagnosis of an arteriovenous malformation and demonstrates its extent, although it is often difficult to distinguish between the actual nidus and the feeding and draining vessels. Trauma is a frequent cause of the growth of the lesion, with hemorrhagic complications, particularly in children and external ear arteriovenous malformations. With clinical examination, midline located arteriovenous fistulas of the forehead can easily be differentiated from sinus pericranii. Associated abnormalities are exceptional, and investigations of the intracranial structures depend on the degree of suspicion.
Treatment must be planned carefully to avoid stimulating progression and interfering with future management. Surgical ligation or endovascular occlusion of proximal feeding vessels must be avoided . Superselective targeted arterial embolization is indicated to decrease symptoms such as pain, bleeding, and ischemic ulceration . Embolization should be performed with permanent agents such as tissue adhesive whenever possible. High-flow cervicofacial arteriovenous malformations are difficult to exclude by arterial embolization alone and should be treated in combination with embolization and surgery. Single-hole arteriovenous fistulas can be easily treated by transarterial embolization. Lesions that are amenable to complete excision are best treated by presurgical embolization and excision . Large lesions involving the skin surface may be well treated with extensive excision and microvascular soft tissue grafting prepared with a tissue expander . Conservative treatment should also be considered for certain lesions.
Intraosseous arteriovenous malformations
Intraosseous arteriovenous malformations are rare and have been overdiagnosed. In most instances, the misdiagnosis results from an erroneous interpretation of bony changes associated with a soft tissue arteriovenous malformation. Soft tissue arteriovenous malformations are often associated with bony defects owing to compression by dilated draining veins. Bony hypertrophy is also seen as a consequence of venous and lymphatic interference related to an adjacent soft tissue arteriovenous malformation. These secondary changes must be distinguished from truly intraosseous arteriovenous malformations.
Mandibular and maxillary arteriovenous malformations
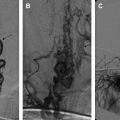
Patients with a maxillary or mandibular arteriovenous malformation often present with life-threatening hemorrhage related to tooth eruption, dental infection, and dental extraction ( Fig. 3 ). This malformation can be managed by arterial embolization followed by extraction of involved teeth . Bone erosion surrounding teeth may be best shown with CT or panoramic radiography. In the authors’ experience, arterial embolization combined with direct injection of tissue adhesive into the intramandibular nidus and draining vein can achieve stable hemostasis and even complete exclusion of the nidus with reossification of the affected mandible. This approach is preferred to mandibulectomy, especially in the immature facial skeleton . Proximal vessel ligation or embolization should be avoided because it leads to recruitment of collateral supply to the lesion and induces nonsprouting angiogenesis indistinguishable from the nidus, which makes subsequent treatment more difficult.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree