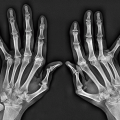
may point to the diagnosis of interphalangeal osteoarthritis; swelling of the proximal and distal interphalangeal joints (Fig. 1.4A) and nail changes such as onycholysis (separation of the nail plate from the nail bed) (Fig. 1.4B), pitting of the nails (Fig. 1.4C), leukonychia (white nails, milk spots) (Fig. 1.4D), splinting hemorrhages (Fig. 1.4E), subungual hyperkeratosis (Fig. 1.4F), and swelling of the entire finger (dactylitis or “sausage digit”) (Fig. 1.4G) are commonly seen in psoriasis; flexible, easy-reducible joint contractures combined with redness and telangiectasia of the nail fold capillaries may be indicative of systemic lupus erythematosus; tapering of the ends of digits and sclerodactyly is characteristic of scleroderma. In the foot, the redness and swelling of the big toe may indicate the presence of gout (Fig. 1.5).
 Figure 1.2 ▪ Rheumatoid arthritis. Observe ulnar deviation in the swollen metacarpophalangeal joints of both hands and hyperextension of the thumbs (“hitchhiker’s thumbs”). |
effusion is in doubt. In most diagnostic and all therapeutic procedures, an attempt should be made to withdraw all fluid accumulated in the joint space. Nonetheless, although about 5 mL is sufficient for most of the necessary studies, as little as one drop may be diagnostic of gout or pyrophosphate dehydrate crystal deposition disease if the appropriate crystals are present (see Figs. 1.9, 1.10, 1.11, 1.12). Furthermore, most studies can be minimized to scale down the amount of drowned fluid in pediatric patients. If there is doubt regarding the origin of fluid from a tap (i.e., blood vs. bloody tap or subcutaneous fluid vs. synovial fluid), this may be resolved with the mucin test or metachromatic staining for the presence of hyaluronate (Table 1.4).
Table 1.1 CATEGORIES OF JOINT FLUID AND DISEASE ASSOCIATIONS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 Figure 1.9 ▪ Synovial fluid—pyrophosphate crystals. Pyrophosphate crystals (blue and red) are identified by polarizing microscopy with interference (compensating) plate in the path of light (H&E, polarized light). (Reprinted from Klein MJ, Bonar SF, Freemont T, et al., eds. Atlas of Nontumor Pathology. Non-neoplastic Diseases of Bones and Joints. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2011, Fig. 8-16, with permission of the author and the Publishers.) |
 Figure 1.11 ▪ Synovial fluid—urate crystals. A: The needle-shaped monosodium urate monohydrate crystals in synovial fluid aspirated from a patient with acute gouty arthritis are viewed here in polarized light with a quarter-wave compensating plate. The slow wave vector (the direction of the extraordinary axis of the compensating plate) is indicated by the arrow. B: In the synovial fluid obtained from another patient with acute gouty arthritis and viewed in polarizing light with an interposed quarter-wave plate, the urate crystals are clearly visible. C: Urate crystals within a neutrophil (Jenner-Giemsa stain). (A and C reprinted from Klein MJ, Bonar SF, Freemont T, et al., eds. Atlas of Nontumor Pathology. Non-neoplastic Diseases of Bones and Joints. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2011, Figs. 8-31 and 8-213, with permission of the author and the Publishers.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|