Doppler Ultrasound of Abdominal Vasculature
Ajay K. Singh
Rathachai Kaewlai
Doppler ultrasound (DUS) examination is a noninvasive means for evaluating the anatomy and blood-flow dynamics of vascular aneurysms, stenoses, or occlusions. It can also guide the interventional radiologist during needle or catheter placement. Understanding the strengths and weaknesses of this modality is crucial for optimal clinical utilization.
Advancements in technology have significantly increased the current role of noninvasive vascular imaging in minimally invasive treatment. DUS provides real-time imaging and physiologic information without exposing the patient to ionizing radiation. This chapter describes six common indications for abdominal DUS related to vascular interventional procedures, namely, abdominal aortic aneurysms (AAAs), transjugular intrahepatic portosystemic shunts (TIPSs), liver transplantation, renal transplantation, and the interrogation of native renal and visceral arteries.
Abdominal Aortic Aneurysm
1. AAA is the most common large-vessel aneurysm encountered in vascular practice. In the United States, routine one-time ultrasound (US) screening is reimbursed for the diagnosis of AAA for men in the age group of 65 to 75 years, who are smokers or have first-degree relatives with AAA.
2. AAA is diagnosed when (a) there is a focal dilation, ≥3 cm or (b) the aortic diameter equals or exceeding 1.5 times its expected normal diameter (1). The majority of AAAs are infrarenal, fusiform, and “true” aneurysms caused by degenerative changes in the aortic wall.
3. Patients with AAA of 4.0 cm or less, 4.1 to 4.5 cm, and 4.6 to 5.0 cm are advised to follow up at 24, 12, and 6 months, respectively. Patients with AAA of 5.1 to 5.5 cm may undergo repair or close follow-up at 3-month intervals. An aneurysm larger than 5.5 cm requires intervention.
a. In nonacute situation, 8 hours of fasting before abdominal DUS is required to reduce the amount of bowel gas.
b. The abdominal aorta is interrogated from the level of aortic hiatus to the bifurcation with visualization of bilateral common iliac arteries. Due to the
depth of the aorta from the anterior abdominal wall, a low-frequency curvilinear transducer is routinely used.
depth of the aorta from the anterior abdominal wall, a low-frequency curvilinear transducer is routinely used.
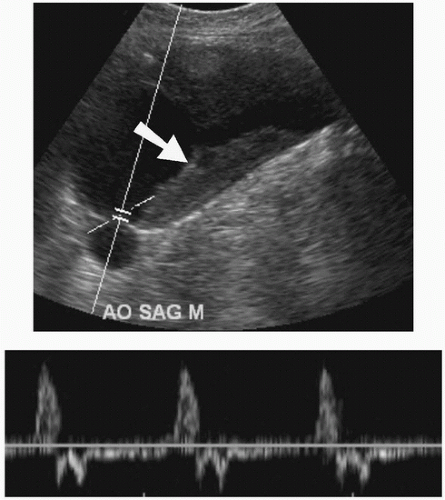 FIGURE e-68.1 • Abdominal aortic aneurysm. Sagittal US view shows an aortic aneurysm with intraluminal thrombus (arrow). Spectral waveform is identified in the lumen of the aorta. |
c. The aorta and common iliac arteries are routinely measured in anteroposterior and transverse diameters from its outer to outer wall. The aorta is measured at the level of the diaphragm, superior mesenteric artery (SMA) origin, and just above the bifurcation. The distance (nonaneurysmal neck) from the renal artery or SMA origin to the most proximal extent of the aneurysm is measured.
5. Lifelong follow-up imaging of aortic grafts or stent grafts is mandatory for detecting possible complications, the two most frequent being endoleak and limb occlusion. Typical follow-up imaging after aortic stent-graft implantation is performed at 1, 6, and 12 months and then at every 1-year interval. In most institutions, computed tomographic angiography (CTA) is considered a gold standard for an evaluation after AAA repair; however, DUS by experienced operators is helpful in patients who cannot receive iodinated contrast agents.
a. Five types of endoleak include type I (poor sealing of either end of the stent with the native aortic wall), type II (retrograde filling of aneurysmal sac via an aortic branch), type III (leak through a defect or tear in the graft),
type IV (seepage of blood into aneurysmal sac due to graft porosity), and type V (significant expansion of the aneurysmal sac without visible leak).
type IV (seepage of blood into aneurysmal sac due to graft porosity), and type V (significant expansion of the aneurysmal sac without visible leak).
b. Limb occlusion is recognized as a lack of flow in the limb(s) of the stent graft.
6. Compared with CTA, DUS has a wide range of sensitivity in detection of endoleak from 64% to 86% with a pooled sensitivity of 77% (2). An endoleak is identified on DUS as color flow outside the endovascular stent graft with a uniform, reproducible, color Doppler appearance that has spectral Doppler waveforms synchronous with the cardiac cycle.
7. Contrast-enhanced US (CEUS) overcomes limitation of US and DUS by providing better delineation of the AAA and detection of endoleak. It enhances characterization of endoleak with analysis of velocity and flow direction. In a meta-analysis (2), its pooled sensitivity was 98% (ranges, 90% to 99%) and pooled specificity was 88% (ranges, 78% to 94%). The European Federation of Societies for Ultrasound in Medicine and Biology currently recommends CEUS for detection and characterization of endoleak and follow-up of known endoleak (3).
Transjugular Intrahepatic Portosystemic Shunt
US is an extremely useful modality to evaluate the hepatic vasculature prior to and after TIPS placement.
1. Prior to the procedure, patency and flow in the portal, splenic, superior mesenteric, hepatic, and internal jugular veins are documented with DUS. The location of the portal vein bifurcation is assessed; undetected variant anatomy can increase the risk of portal vein perforation into the peritoneal cavity during the TIPS procedure.
2. After TIPS placement, DUS is routinely performed within 24 to 48 hours, at 3 months, and then at every 6 months to assess shunts patency and function. The TIPS stent appears as two parallel curvilinear lines, with corrugated appearance to the wall, connecting the hepatic and portal veins (Fig. e-68.2).
Although a typical TIPS stent is 10 mm in diameter, its in vivo diameter is 8 to 9 mm due to surrounding hepatic tissue recoil (4). The portal venous and hepatic venous ends of the stent are normally slightly flared and should be within the lumen of hepatic and portal veins, respectively. A fabric-covered stent may contain some gas (air) that causes shadowing for several days until the gas is absorbed. The spectral waveform within TIPS



Although a typical TIPS stent is 10 mm in diameter, its in vivo diameter is 8 to 9 mm due to surrounding hepatic tissue recoil (4). The portal venous and hepatic venous ends of the stent are normally slightly flared and should be within the lumen of hepatic and portal veins, respectively. A fabric-covered stent may contain some gas (air) that causes shadowing for several days until the gas is absorbed. The spectral waveform within TIPS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





