Ductal Carcinoma In Situ and Early-Stage Breast Cancer: Detection, Diagnosis, and Prognostic Indicators
Radiologists have taken pride in the fact that mammographic screening has led to the detection of the earliest form of breast cancer—ductal carcinoma in situ (DCIS). Mammography is the only way that small, noninvasive breast cancers can be detected. Before 1984 it was exceedingly rare for DCIS to be detected. When it was, it was almost always an extensive lesion that was palpable. In 1984 there was a sudden increase in the detection of DCIS as indicated in the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This marked the date when mammography screening began to be used by a sufficient number of women to be reflected in national statistics.
What seemed like a victory to some was seen by others as a major problem. This was articulated in a 1996 article in the Journal of the American Medical Association in which Ernster et al (1), rather than praising the ability of mammography to detect preinvasive lesions, faulted the screening test for its ability to detect these lesions. They cited the fact that the increased rate of detection of DCIS that occurred with the introduction of mammography screening had led to “overtreatment” of women. They raised the legitimate concern that these lesions were not clearly cancer, since they had questionable lethal potential. Nevertheless, as the authors pointed out, these early lesions, of uncertain significance, were being treated by mastectomy at a time when women with potentially lethal, invasive cancers were having their breasts preserved by lumpectomy and radiation. Although this concern was legitimate, it was a case of “shooting the messenger.” Radiologists were being faulted for finding these lesions when in fact it was the pathological interpretation and inappropriate therapy that had not caught up with the ability of technology to find these lesions. Nevertheless, important questions were raised concerning DCIS and its significance.
There is no question that mammography is, essentially, the only way to detect most DCIS lesions. Before the widespread use of mammographic screening, approximately 2% to 5% of all cancers that were diagnosed each year were DCIS (2). At present between 20% and 30% of cancers detected in the United States are DCIS (3). Many believe that the detection of breast cancer in its earliest form must be beneficial, but that is not so clear. If DCIS is the precursor lesion for all invasive cancers, then the detection of these
lesions should interrupt the progression to invasion and the number of invasive cancers should decrease as the number of DCIS lesions increases. It is perplexing that this has not happened. Despite the detection of 20,000 or more cases of DCIS each year, the number of invasive cancers does not appear to have decreased. Part of the problem may be that because the rate of invasive cancers has been increasing steadily each year since 1940, it might be difficult to detect a relative decrease; perhaps the rate of increase would have been greater had we not been finding DCIS. Nevertheless, the fact remains that there is little direct evidence that the detection of more DCIS has been beneficial. On the other side of this confusing situation is the fact that when DCIS lesions are excised, if the breast is not treated with irradiation, as many as 24% will recur within 5 years, and half of these will recur as invasive cancers (4). The natural history and significance of these lesions remain confusing and uncertain.
lesions should interrupt the progression to invasion and the number of invasive cancers should decrease as the number of DCIS lesions increases. It is perplexing that this has not happened. Despite the detection of 20,000 or more cases of DCIS each year, the number of invasive cancers does not appear to have decreased. Part of the problem may be that because the rate of invasive cancers has been increasing steadily each year since 1940, it might be difficult to detect a relative decrease; perhaps the rate of increase would have been greater had we not been finding DCIS. Nevertheless, the fact remains that there is little direct evidence that the detection of more DCIS has been beneficial. On the other side of this confusing situation is the fact that when DCIS lesions are excised, if the breast is not treated with irradiation, as many as 24% will recur within 5 years, and half of these will recur as invasive cancers (4). The natural history and significance of these lesions remain confusing and uncertain.
Does DCIS Progress to Invasive Cancer?
The Ernster critique was followed by an article by Welch and Black that attempted to reinforce the contention that most cases of DCIS were unimportant: they estimated that at most only 25% of DCIS lesions would ever progress to possibly lethal lesions (5). They based their argument on the fact that in several autopsy series DCIS had been found among women who died of other causes (6). In addition, several autopsy studies have found undetected DCIS and invasive cancers in the breast tissues of women who died accidentally (7,8). Clearly these lesions had never affected the women during life, and so they argued that many lesions detected by mammography were nonthreatening. This turned out to be an incorrect assumption. Certainly among older women who were incidentally found to have breast cancer at autopsy (the cancer never affected their lives), the detection of these cancers, while the individual had been alive, would only have been harmful by causing unnecessary psychological trauma and totally unnecessary treatment (9). Welch and Black, however, failed to understand the significance of cancers detected at autopsy of women who died accidentally. These represent a very different group. These are not necessarily innocuous lesions. What has been confused is that undiscovered cancers in “older” women may prove to be inconsequential, but undiscovered invasive and in situ cancers in women who die accidentally may be more important. The “forensic” studies (premature deaths) do not look at the prevalence of cancers at the end of life as among older women, but rather they provide a window into the prevalence of potentially lethal cancers that are growing in the population. The fact that these women died “accidentally” is similar to having randomly selected them. The accidental deaths data represent a true slice through the population. There is no reason to assume that the lesions found in these women would not have become relevant cancers had the women led full lives. In other words, rather than showing the distribution of breast cancer in older women at the end of life, the amount of cancer found among women who die prematurely, by accident, represents a cross-section of the pool of cancers that are present during the early and middle years of life. These are the cancers that contribute to the incidence of malignancies that are discovered each year. Cancers among women who died accidentally represent the true prevalence of undiscovered breast cancer in the population.
Welch and Black concluded from these studies that, among women aged 40 to 49, at most only 25% of DCIS cases were likely to become a possibly lethal lesion (10). They reasoned that because 12% of women who died accidentally had DCIS but only 3% of women who die each year die of breast cancer, only 25% of DCIS lesions could progress to lethal cancers (3%/12% = 25%). Their major mistake was that they compared the amount of DCIS found in a random cross-section of the population (women who died accidental deaths) to the risk of dying from breast cancer each year in the entire population. This was not a valid conclusion because cross-sectional data are a measure of the prevalence of cancer in the population, whereas the death rate relates to the annual incidence of breast cancer. Welch and Black’s comparison between the amount of breast cancer that appears to be present in the total population (as reflected by cancers found in the women who died accidentally) and the number of deaths from breast cancer each year in the total population would be accurate only if cancer progressed from one cell to killing the individual in a single year. Comparing the amount of breast cancer present in the population to the number of deaths each year provides no useful measure of the risk from the undiagnosed cancers. However, the data from women who died accidentally do provide a measure of the true amount of breast cancer that is in the population.
To try to better understand the autopsy data, we developed a simple model of breast cancer growth (11). The model does not prove that DCIS becomes invasive breast cancer, but it does explain why, even if all DCIS lesions become invasive cancers, we would expect to find a much large reservoir of undetected lesions, at any given time, in the population than are detected each year (helping to explain the autopsy findings). Assume that we can find only cancers that reach 2 cm in size. If a cancer starts as a single cell in a duct, its clones grow in that duct as DCIS for 10 years, and then an invasive clone develops and that clone doubles every 180 days, it would take approximately 30 years for the single DCIS cell to result in a 2-cm invasive tumor. If it is assumed that a new cancer starts this way each year, and that we can only detect 2-cm invasive cancers, for every lesion that reaches the 2-cm size there will be 20 smaller invasive cancers and 10 DCIS lesions that are growing in the population undetected. This would be the case if all the DCIS lesions ultimately become 2-cm invasive
cancers. The number of DCIS lesions becomes even larger if some do not become invasive cancers. This estimate is for a slow-growing cancer, but the calculation can be made for faster-growing cancers. The number of DCIS lesions growing in the population will equal the number of years it takes for the DCIS lesion to become invasive, and the number of invasive cancers growing undetected in the population will equal the number of years it takes for an invasive lesion to go from the first cell to a detectable size. Regardless of the numbers, there are clearly many more cancers growing at any time in the population than the number of cancers being detected each year. There is as yet no way of telling which of these lesions could become lethal.
cancers. The number of DCIS lesions becomes even larger if some do not become invasive cancers. This estimate is for a slow-growing cancer, but the calculation can be made for faster-growing cancers. The number of DCIS lesions growing in the population will equal the number of years it takes for the DCIS lesion to become invasive, and the number of invasive cancers growing undetected in the population will equal the number of years it takes for an invasive lesion to go from the first cell to a detectable size. Regardless of the numbers, there are clearly many more cancers growing at any time in the population than the number of cancers being detected each year. There is as yet no way of telling which of these lesions could become lethal.
The reservoir of DCIS lesions cannot be used to predict what percentage become invasive, but Welch and Black’s hypothesis that a large reservoir means that only a small percentage become invasive is not supported. It is likely that there are many invasive cancers that go through a very short DCIS phase; indeed, some cancers may be invasive from the second cell. Nevertheless, the gross observations would remain the same, and the reservoir of tumors in the population (DCIS and invasive) should be expected to be much larger than the number of cancers detected each year even if all become detectable and all are biologically relevant. Only if we are able to dive way down in the “pool” and detect cancers as they are being initiated will they approach equality.
Trying to determine whether DCIS, if left alone, will progress to an invasive lesion is complicated by the fact that to make the diagnosis, the lesion must be biopsied. Needless to say, this alters its natural history. One might think it possible to study the natural history of DICS by looking at women who had lesions that were biopsied and mistakenly called benign and not treated. However, some of the lesions may have been completely removed, so that following up the women might underestimate the number that progressed to invasive cancers. This likely compromises retrospective studies that analyzed low-grade lesions that were misdiagnosed as benign years ago and therefore not treated. The same is of course true for the randomized controlled trials that evaluated treatment using radiation and tamoxifen. This problem also compromises extrapolation using registry studies where the lesions are excised, with no additional treatment. In these studies the effort is to actually try to remove the entire lesion. It is not possible to accurately extrapolate the underlying risk based on recurrence rates following these procedures. As a consequence, the true course of DCIS is unknown. There are no direct measurements that can be made to determine what percentage of DCIS lesions, if left untouched, would progress to invasive cancers.
DCIS is Not A Single Lesion
Our understanding of DCIS is further complicated by the fact that there is not just one form of these early lesions. Based on histologic criteria, pathologists now divide DCIS into three categories. Grade I DCIS is thought to be fairly indolent and unlikely to invade for many years. High-grade (grade III) DCIS is thought to be more aggressive and likely to invade early. Intermediate-grade lesions are in between (12). Since it is fairly universally agreed that cancer confined to the ducts cannot be lethal, the real question is which of these lesions will progress to become invasive cancer. Using the results for the Swedish Two-County randomized controlled trial of screening, Duffy et al estimated that at the first screening study, 37% of DCIS lesions would not progress to invasive cancers (13). On subsequent screens that detected new cases of DCIS, only 4% would not progress. By estimating how many cases that would have been stage 1 or 2 cancers had they not been detected as DCIS, they believed that approximately 12% of the decrease in deaths that occurred in that trial was due to the detection of DCIS.
Pathologists now seem to agree that there are distinct subtypes of intraductal cancer. There is still debate as to how best to classify these lesions (14). Many see a difference based on cell morphology and the architecture of the aggregated cells. Large cell tumors appear to have a different natural history than small cell cancers. Solid growth (cells abutting one another with no defined spaces within the tumor) is distinguished from those forming cribriform (sieve-like) spaces. Many tumors have areas of necrosis; others have micropapillary growths.
A consensus has emerged that divides DCIS into three categories: poorly differentiated (high grade), intermediate differentiation, and well differentiated (low grade). High-grade DCIS is characterized by relatively large, pleomorphic cells with large nuclei (more than two red cell diameters), whereas low-grade DCIS consists of monotonous, relatively small cells that are similar in morphology.
Lagios et al (15) were one of the first groups to develop a histologic grading system for DCIS that some believe can be used to predict the growth patterns of these lesions. They found that poorly differentiated DCIS, particularly of the comedo (large cell) type, appears to recur early (within 5 years of discovery), often with invasion. They noted that micropapillary or cribriform types progressed at a slower rate. Their grading system for DCIS categorized the cytologic features of the lesions.
Initially Lagios et al used a classification system that was the reverse of the system used for invasive lesions. Grade I was the highest or worst grade, whereas grade IV was the lowest, indicating the best prognosis. Lagios’s grade I was a poorly differentiated large cell lesion, with comedo necrosis. Grade II was a small cell type with a cribriform pattern but with associated necrosis. Grade III was a cribriform pattern with anaplasia. Grade IV was the small cell, micropapillary form of DCIS.
Lagios’s data suggested that it might be possible to predict
which lesions were likely to recur within a few years and which were likely to not recur for many years, if at all (16). This could have a significant impact on therapeutic interventions. In a study of 79 women treated for DCIS by excision alone in which the tumor was less than 2.5 cm in its greatest extent, Lagios reported an overall recurrence rate of 10% at an average follow-up of 4 years. In this series, necrosis was an important prognostic feature. Five of the 31 (16%) lesions of the large cell, comedo type of DCIS recurred within 3 years of diagnosis, having been treated by excision alone, and an additional 2 of 5 (40%) of the small cell cribriform cancers with necrosis also recurred. Only 1 of 10 (10%) of the cribriform cancers without necrosis recurred, and none of the 33 micropapillary lesions had recurred after 6 years of follow-up.
which lesions were likely to recur within a few years and which were likely to not recur for many years, if at all (16). This could have a significant impact on therapeutic interventions. In a study of 79 women treated for DCIS by excision alone in which the tumor was less than 2.5 cm in its greatest extent, Lagios reported an overall recurrence rate of 10% at an average follow-up of 4 years. In this series, necrosis was an important prognostic feature. Five of the 31 (16%) lesions of the large cell, comedo type of DCIS recurred within 3 years of diagnosis, having been treated by excision alone, and an additional 2 of 5 (40%) of the small cell cribriform cancers with necrosis also recurred. Only 1 of 10 (10%) of the cribriform cancers without necrosis recurred, and none of the 33 micropapillary lesions had recurred after 6 years of follow-up.
Silverstein et al (17) used a similar grading system that they found predicted local recurrence and disease-free survival. Their grade I lesions consisted of DCIS in which the cells had low nuclear grade with nuclei that were 1 to 1.5 red blood cells in diameter with diffuse chromatin and no visible nucleoli. Their grade 2 lesion had cells in which the nuclei were larger (one to two red cell diameters), with coarse chromatin and occasional nucleoli. Their grade 3 (high grade) had nuclei that were greater than two red blood cell diameters with “vesicular” chromatin and one or more nucleoli. Using these parameters, they classified their lesions. Their group 1 consisted of lesions that had nuclear grade 1 or 2 but did not demonstrate any necrosis. Group 2 lesions were grade 1 or 2 but with necrosis, and group 3 were the high-grade lesions. Among 238 patients, there were 31 local recurrences. Only 3 recurred out of the 80 lesions classified as well differentiated, group 1 (3.8%). Among the 90 women classified as having group 2 lesions (moderately well differentiated), 10 recurred (11.1%), whereas there were 18 recurrences in the 68 group 3 lesions (26.5%) (poorly differentiated). The 8-year actuarial disease-free survivals using the Van Nuys system were 93% for group 1, 84% for group 2, and 61% for group 3.
Holland et al (14) developed a similar grading scheme. They divided DCIS into poorly differentiated, intermediate (or moderately differentiated), and well-differentiated lesions. Their classifications are based on cellular features as well as patterns of growth. These features include nuclear grade, architectural relationship of cells, and presence or absence of necrosis. The nuclear morphology in the cells is a primary prognostic indicator. Some of the features they suggest should be used to classify DCIS are listed in Table 7-1.
As in the other systems, their preliminary data suggest that poorly differentiated lesions recur early, with a high percentage of the recurrences being invasive cancers. Well-differentiated DCIS is slow to recur, taking 10 or more years, and invasion is less common. It has also been suggested that when an invasive cancer is poorly differentiated, any associated DCIS is poorly differentiated, and low-grade invasive lesions tend to be associated with low-grade DCIS.
TABLE 7-1 HISTOLOGIC GRADING OF DCIS | ||
|---|---|---|
|
The long-term success of these grading systems remains to be seen, as well as how they will be used in the management of DCIS. Some argue that it is only a matter of degree and that ultimately, given sufficient time, many of these lesions, regardless of their levels of differentiation, will recur if not treated completely, and many will develop invasive capability.
The importance of long-term follow-up is apparent in a review by Page et al (19). They followed 28 patients whose previous biopsies had been incorrectly diagnosed as benign. On histologic review, the biopsied lesions were retrospectively diagnosed as low-grade cribriform DCIS. Among these 28 women, there were nine (36%) recurrences over a 25-year period. Seven of the women (25% of the total and 78% of the recurrences) developed invasive cancers within 10 years in the same quadrant of the same breast. The two other recurrences developed in subsequent years. There were five breast cancer deaths (18%) among this group. This clearly shows that even low-grade DCIS is not a totally innocuous lesion. The potential importance of DCIS is further suggested by the fact that studies such as this evaluate lesions that have been excised, and some were probably completely excised. Thus, long-term follow-up
of excised DCIS probably underestimates the risk from DCIS that is left alone. If a patient has a significant life expectancy, even low-grade DCIS can be an important lesion.
of excised DCIS probably underestimates the risk from DCIS that is left alone. If a patient has a significant life expectancy, even low-grade DCIS can be an important lesion.
What is Breast Cancer? Survival from Even Invasive Cancers is Better Than Most Realize
Despite centuries of research, there is no universal agreement over the definition of breast cancer. Clearly the most important question to be answered about a breast lesion is its potential to kill. Fortunately, many if not most women who develop breast cancer, even invasive lesions, do not die of the malignancy. Even in the years before earlier detection, many women did not die of their breast cancers. Adair et al looked at 1,640 women diagnosed with breast cancer between 1940 and 1943 (18). Among these, 182 (11.1%) presented with such advanced disease that they were deemed inoperable. They already had distant metastatic disease, inflammatory cancer, supraclavicular adenopathy, or nonresectable tumor with fixed, immovable axillary nodes. The remaining 1,458 women were followed up at 30 years. Eighty-four women (13%) were alive a minimum of 28 years after treatment. A little over half (826) had died of breast cancer (57%). Approximately a quarter (349) had died of other causes (24%) and 99 (6.8%) were lost to follow-up. It is of interest that 100 developed contralateral breast cancer (7%). The researchers estimated that even in the era before screening and adjuvant treatment, there had been a 35% overall actuarial 30-year survival (even women with positive nodes [60/184] were alive at 28 years).
Since the incidence of breast cancer increases with increasing age, many women who are diagnosed with breast cancer and are of advanced age die from other causes, such as heart disease. Even among younger women, those diagnosed with breast cancer are more likely to survive the cancer than they are to be killed by it. For decades the data show that only approximately 40% of women who are diagnosed with invasive breast cancer ultimately die of the cancer. It is not clear whether this is due to earlier detection and better treatment or merely because some lesions that are classified as cancers have the potential to be lethal but others do not. The threat of death from some lesions, such as well-differentiated DCIS, may be extremely remote (19), whereas mortality from a rapidly growing, poorly differentiated invasive cancer may be more immediate. Although there is hope that molecular characteristics will be able to predict future lethality (20), for any specific lesion there is as yet no way to predict outcome with any certainty.
What is The Appropriate Biologic Model for DCIS?
There are two schools of thought with respect to the development of breast cancer. One school believes that breast cancer is the end result of a series of changes forming a continuum. This was first postulated by Gallager and Martin, who based their conclusions on observations from extensive whole-breast histologic analysis (21). Their observations suggested a logical progression of epithelial transition to cancer. They showed that most breast cancers originate in the intralobular terminal duct. According to their studies, normal ductal epithelium in this region can undergo reversible hyperplastic growth. In some women, the cells begin to have atypical features. They believed that atypical epithelial hyperplasia was the last reversible change that precedes the irreversible transition to carcinoma in situ, which ultimately progresses to invasive breast cancer. Once invasive, a cancer can gain access to the lymphatic and vascular systems and spread to other organs, ultimately killing the individual.
Others, however, believe that breast cancer is not part of a continuum but represents a duality of growth. They would argue that only invasive breast cancer is a true cancer and that it arises de novo without going through an in situ phase and that in situ breast cancer is of little consequence.
The continuum theory would compare breast cancer to cancer of the cervix, which, after a variable in situ period, becomes invasive. The dual theory likens breast cancer to cancers of the thyroid and prostate. In these organs there appear to be histologically evident cancers that remain confined locally and are not lethal and other cancers that invade rapidly and metastasize early.
Although prostate cancer has been compared to breast cancer, there are significant differences. It is likely that most cancers of the prostate develop so late in life that other causes of death intervene before many prostate cancers become significant. In contrast, breast cancer affects women a decade or more earlier than prostate cancer affects men, making it more likely that early breast cancer lesions will become significant.
Although there is hope that molecular assays will be able to subcategorize cancers into those that will be lethal and those that will be indolent (20), these are still in the early years of exploration and it remains to be shown how well they will predict lethality and indolence. The inability to accurately predict the course of a given lesion may merely indicate that the analytic tools available today cannot distinguish between cancers with lethal potential and those that will not result in death. However, it is also possible that just as chance mutations or DNA damage likely lead to cancer in the first place, the course of a cancer may be altered by subsequent DNA changes that are not predictable. Until we can accurately predict the future for a given lesion, it is prudent to treat most breast cancers as if they have lethal potential. However, this clearly does not mean a mastectomy is needed. Studies are needed to determine the best treatment for each type of lesion.
The randomized controlled trials of screening have shown that the progression of breast cancer need not lead inexorably to death. The natural history (whatever it might be)
of some cancers can be interrupted and death averted through earlier detection. What remains to be determined is how best to treat the various manifestations of breast cancer to maximize the benefit while minimizing the harm to the individual from detection, diagnosis, and treatment.
of some cancers can be interrupted and death averted through earlier detection. What remains to be determined is how best to treat the various manifestations of breast cancer to maximize the benefit while minimizing the harm to the individual from detection, diagnosis, and treatment.
It is well established that prognosis is directly related to (although not absolutely determined by) the size of a cancer (22), its histologic grade, and whether tumor cells have spread to the axillary lymph nodes (reflecting metastatic capability) (23). The detection of breast cancer earlier in its growth has clear advantages, and the purpose of this chapter is to review the present understanding of what constitutes “early” breast cancer. There are likely multiple paths to invasive breast cancer. Some likely arise from a single cell and are fully capable of invasion and metastatic spread very early in their growth, if not immediately; others likely develop in epithelium that is extensively altered and genetically unstable, with changes occurring over long periods of time and resulting in one cell ultimately accumulating the genetic changes needed for malignant transformation.
Breast Cancer and Its Subcategories
Breast cancer is not a single disease process; it has numerous variations. Furthermore, the outcome for an individual is determined not merely by the cancer but also by the host’s response to the cancer. Our ability to classify cancers remains crude. Pathologic review of the tissues using light microscopy remains the most accurate diagnostic test. Pathologists have subclassified cancers of the breast, and it is important to understand their terminology and classification.
Ductal Carcinoma (In Situ and Invasive)
A very small percentage of malignant lesions arise from the stromal elements of the breast. Ninety percent of breast cancers have cellular features that are similar to ductal epithelium and are consequently classified as ductal cancers. When in situ (remaining confined to the duct), they are called intraductal carcinoma, or more commonly DCIS. When the cells have breached the basement membrane surrounding the duct and invaded the surrounding tissues, they are termed invasive or infiltrating ductal carcinoma.
Because most breast cancers arise in the ducts, the earliest manifestation of breast cancer is DCIS. Because cancer that is confined to the ducts cannot spread beyond the breast and cannot be lethal, as noted earlier, it is not clear whether DCIS actually progresses to invasive cancer. Thus, DCIS is often considered separately in cancer statistics. Some do not even consider it to be a true malignancy. The data, however, suggest that DCIS is directly related to invasive breast cancer. Virtually all invasive cancers that develop in women who have had DCIS grow in the same location as the DCIS. It is common for invasive cancers to be intimately associated with DCIS. Although not all cancers may have recognizable areas of DCIS, this lesion is almost certainly a direct precursor lesion for many if not all invasive cancers.
At present it is impossible to resolve disagreements over the natural history of breast cancer and the importance of in situ cancer. The diagnosis of intraductal carcinoma can be made only using biopsy. The process of making the diagnosis alters the course of the lesion, and an accurate study of its natural history is impossible. One study suggests that DCIS lesions, if biopsied but left without definitive treatment, will progress to invasive cancer in at least 30% to 50% of women (24).
A study conducted by the National Surgical Adjuvant Breast Project (NSABP), a large multicenter collaborative group, found that women with DCIS (not further categorized) treated with excision alone had a 23% recurrence rate by 3 years, whereas those treated with excision and radiation had the recurrence rate reduced to 9% (25). A larger NSABP study involving only women with mammographically detected DCIS confirmed this finding. Among 818 women with DCIS randomized to be treated with lumpectomy alone or lumpectomy and radiation therapy to 50 Gy (5,000 Rads) with a mean follow-up of 43 months (range 11 to 86 months), they found that among the 391 women treated by lumpectomy alone, 64 (16.4 percent) developed a recurrent cancer in the same breast. Of these, 32 (50%) of the recurrences were noninvasive and 32 (50%) women developed invasive recurrences. Among the 399 women treated with lumpectomy and breast irradiation, 28 (7%) developed recurrences in the same breast: 20 (71%) of these had noninvasive recurrences and only 8 (28%) had invasive recurrences. The 5-year cumulative incidence of second cancers in the ipsilateral breast was reduced by irradiation from 10.4% to 7.5% for noninvasive cancers and from 10.5% to 2.9% for invasive cancers (P = 0.055 and P < 0.001). The fact that in situ recurrences were reduced by adding irradiation was important, but of even greater importance was that 50% of the recurrences in the nonirradiated group were invasive cancers (8% overall by 4 years) and two women died of breast cancer (26). Adjuvant radiation cut the recurrence rate for invasive breast cancer in half.
This study was again updated in 1999 (27). With longer (8 years) of follow-up, it was found that radiation following lumpectomy reduced the recurrence rate in the breast from 31% to 13%. Although 40% of the cancers that recurred were invasive, mortality was still only 1.8% at 8 years.
The importance of and the need for radiation therapy in patients treated for DICS has been challenged, however. Silverstein and Lagios (28) and others have argued that if the lesions are properly evaluated and sufficient normal tissue is removed around the DCIS (DCIS is virtually always a process confined to a lobe or segment of the breast) so that the margins of the tissue are free of tumor, many low-grade lesions can be treated with excision alone. It is my
impression that the kind of excision advocated by Silverstein is far more extensive than merely excision to negative margins. It requires extensive surgery and, by Silverstein’s own admission, plastic surgery-like procedures (“oncoplastic surgery”). The extensive surgery involved in the Silverstein approach makes it unlikely to be acceptable to most women and their surgeons, and I suspect that lumpectomy to clear margins (2 mm free of tumor) followed by irradiation will be the standard treatment for DCIS until more accurate predictors of recurrence can be determined.
impression that the kind of excision advocated by Silverstein is far more extensive than merely excision to negative margins. It requires extensive surgery and, by Silverstein’s own admission, plastic surgery-like procedures (“oncoplastic surgery”). The extensive surgery involved in the Silverstein approach makes it unlikely to be acceptable to most women and their surgeons, and I suspect that lumpectomy to clear margins (2 mm free of tumor) followed by irradiation will be the standard treatment for DCIS until more accurate predictors of recurrence can be determined.
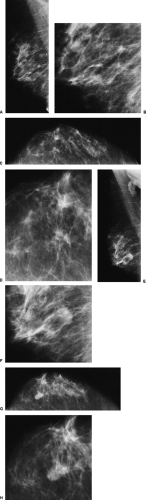
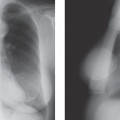
These results can also be interpreted in two other ways. Those supporting the dual theory would say that at most 50% of intraductal cancers ever become significant, whereas the continuum proponents might suggest that if lesions are excised completely and early, their progression may be interrupted and the patient cured of the disease. Radiologists are, unfortunately, well aware of examples of lesions that were almost certainly DCIS that were overlooked or left untreated and progressed to invasive breast cancer (Fig. 7-1). Such anecdotal cases should serve as a reminder and not be ignored, but scientific studies should dictate care.
The data strongly suggest that DCIS does represent a precursor of invasive breast cancer. There has never been a reported case of “cancer calcifications” that disappeared unless they were replaced by an invasive lesion. As many
point out, virtually all cancers originate in the ductal epithelium and thus are by definition intraductal at the start. It is may be that if the individual lives long enough, DCIS will often progress to invasion. There are no clear studies to determine the actual rate of progression. Based on data from the Swedish Two-County screening trial, Duffy et al have estimated that most of the deaths (65%) that were averted in that mammography screening trial were the result of downstaging that occurred by finding cancers at stage I, before they grew to stage II (29). In their trials they estimated that approximately 12% of deaths that were avoided may have been avoided due to the detection of DCIS. However, in another review of the Two-County data, the authors concluded that more than 60% of DCIS lesions
will progress to invasive cancers (30). This apparent contradiction is just a reflection of the fact that many invasive breast cancers are not lethal.
point out, virtually all cancers originate in the ductal epithelium and thus are by definition intraductal at the start. It is may be that if the individual lives long enough, DCIS will often progress to invasion. There are no clear studies to determine the actual rate of progression. Based on data from the Swedish Two-County screening trial, Duffy et al have estimated that most of the deaths (65%) that were averted in that mammography screening trial were the result of downstaging that occurred by finding cancers at stage I, before they grew to stage II (29). In their trials they estimated that approximately 12% of deaths that were avoided may have been avoided due to the detection of DCIS. However, in another review of the Two-County data, the authors concluded that more than 60% of DCIS lesions
will progress to invasive cancers (30). This apparent contradiction is just a reflection of the fact that many invasive breast cancers are not lethal.
There is no question that the reservoir of DCIS in the population is considerably higher than the number of invasive cancers reaching the surface (being detected) each year. In our model we showed that for every invasive lesion that reached 2 cm in size each year, there could be as many as six DCIS lesions in the population “below the surface” and undetected, even if all DCIS lesions were to progress to invasive cancer. If only a percentage progress, but all cancers begin as DCIS, then the number of undetected cases of DCIS would be even larger. The question that remains is what percentage of these in situ lesions will progress to invasive, lethal lesions.
An Opportunity to Evaluate the Natural History of DCIS
Because most cases of DCIS detected in the United States are surgically removed, the natural history of the lesion is always interrupted. The European screening trials provide an opportunity to develop a better understanding of the natural history of DCIS. In many of the trials the thresholds for intervention have been higher than those in the United States. The borderline clusters of calcifications that are excised in the United States were likely ignored in some of the European trials. A review of the invasive breast cancers that were eventually diagnosed in those trials might reveal which patients had calcifications indicative of DCIS that were allowed to pass through an earlier screening. Unfortunately, pride (few wish to admit that they permitted cancer to pass through a screen) and the cost of such a review will likely prevent the undertaking.
Other factors will likely be discovered to help predict the natural history of DCIS. Investigators continue to elucidate the DNA sequences involved in the various stages of breast cancer development. The expression of several genes, such as HER2, has already been linked to a more aggressive form of DCIS, and more details will be revealed in the future. Grading DCIS and ultimately DNA analyses may provide the patient and her physician with a better understanding of the potential virulence of these lesions.
Microscopic Invasion
As noted earlier, pure DCIS cannot be lethal. The only way that breast cancer can kill is through metastatic spread. Analysis of the significance of DCIS is further complicated because the pathologist’s differentiation of in situ from invasive lesions may be inaccurate because he or she can review only a minute portion of the entire tumor. It would require thousands of slides to evaluate every level through a tumor to identify where it might have broken through the basement membrane and invaded into the stroma. This sampling error accounts for the fact that invasion may be overlooked.
Some lesions that appear confined to the ducts by light microscopy have been shown on electron microscopy to have transgressed the basement membrane into the surrounding stroma (31). This may explain why a few women with apparent intraductal carcinoma are paradoxically found to have tumor in their axillary lymph nodes when the diagnosis is made, and some actually die of metastatic breast cancer. The likelihood of “microscopic” invasion increases with the size of the DCIS lesion. On careful sectioning, Lagios et al found that if the DCIS lesion was larger than 2.5 cm, there was almost a 50% (11/24) probability that there was microscopic invasion (32). There was no microscopic invasion for any lesion smaller than 25 mm. For most mammographically detected DCIS lesions, which are usually very small, invasion is very rare. The likelihood of nodal metastasis does increase when microinvasion is present. Overall, only 2% of women with DCIS who have
axillary dissections will be found to have tumor in their lymph nodes. For this reason, axillary node dissection is rarely indicated for DCIS, although some surgeons are now performing sentinel node biopsies for women with large volumes of DCIS, especially if it is poorly differentiated.
axillary dissections will be found to have tumor in their lymph nodes. For this reason, axillary node dissection is rarely indicated for DCIS, although some surgeons are now performing sentinel node biopsies for women with large volumes of DCIS, especially if it is poorly differentiated.
Might Some Lesions that Look Like DCIS Actually Represent Invasive Cancers that Are Forming What Look Like Ducts?
Separately, Tabar and Igleheart have raised another possibility as to why some DCIS lesions or small invasive lesions with a large DCIS component have a much higher rate of lethality than expected. Both have suggested to me that some cancers that appear to be cases of extensive DCIS actually represent invasive cells that build structures that look like ducts filled with DCIS. Tabar et al have shown that small invasive cancers that are associated with extensive DCIS that is necrotic with linear branching calcifications (called “casting” by Tabar) have a much worse prognosis than the small amount of apparent invasion would predict (33). In the long-term follow-up of women in the Swedish Two-County screening trial, the survival of women who had invasive cancers that were 1 to 9 mm in size with no casting-type calcifications was about 95%. However, 14% of 138 women who presented with very small, 1- to 9-mm tumors that had associated casting-type calcifications on mammography accounted for 73% of all breast cancer deaths (p




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree