Abstract
Friedreich’s ataxia (FRDA) is a neurodegenerative disease characterized by progressive ataxia, dysarthria, sensory loss. While neurological symptoms are prominent, cardiac manifestations significantly contribute to mortality. Cardiomyopathy in Friedreich’s disease results from mitochondrial dysfunction, loss of contractile proteins and an accumulation of fibrosis in heart. To better characterize the severity of cardiac involvement, the MICONOS study group developed a classification system categorizing FRDA cardiomyopathy as “no,” “mild,” “intermediate,” “severe.”
We report an uncommon case of early-onset development of hypertrophic cardiomyopathy (HCM) in a 25-year-old female diagnosed with Friedreich’s ataxia (FRDA) at age 12. Through annual cardiac evaluations, no signs of cardiac disease were noted. Until presenting with dyspnea and palpitations. Clinical examination revealed truncal ataxia and dysarthria, but no signs of heart failure. However, a transthoracic echocardiography demonstrated nonobstructive hypertrophic cardiomyopathy with a maximal wall thickness of 20 mm, incomplete anterior systolic motion of the mitral valve, a significant development in less than 1 year after last normal cardiac assessment. Left ventricular systolic function was preserved (ejection fraction 50%). She was prescribed bisoprolol and dapagliflozin, with significant improvement at her latest checkup. Family screening revealed HCM in her 30 year female sibling, who also has FRDA. No cardiac abnormalities were detected in her younger brother or parents.
Friedreich’s hypertrophic cardiomyopathy has been reported as the most significant cause of mortality, especially among younger patients with early onset disease manifestations.
Introduction
Friedreich’s ataxia (FRDA) is a neurodegenerative disease with autosomal recessive inheritance pattern, caused by a lack of a protein called frataxin (FXN), which is essential for mitochondrial function [ ]. Patients with FRDA experience progressive ataxia, dysarthria, sensory loss, and pyramidal signs [ ].
While the progressive, irreversible ataxia and neuromuscular deterioration associated with FRDA are widely acknowledged, significant mortality arises from cardiomyopathy [ ]. Cardiac manifestations are prevalent among FRDA patients and is considered a considerable factor to premature mortality. Historically categorized as a hypertrophic cardiomyopathy, the cardiac phenotype of FRDA is increasingly recognized for its heterogeneity. For instance, prior investigations have indicated that the hypertrophic heart in FRDA patients may undergo a transition to a dilated state [ ], and the MICONOS study group had proposed a severity classification of FDRA’s cardiomyopathy into “no”, “mild”, “intermediate” and “severe” [ ].
In this article, we report an uncommon case of familial hypertrophic cardiomyopathy development in a female patientdiagnosed with Friedrich’s Ataxia.
Case presentation
This case involves a 25 year old female patient with a medical history of Friedrich’s ataxia, the first manifestations of the disease begun early at the age of 12 years old, progressively the patient presented symptoms of cerebellar ataxia, dysarthria, and muscle weakness of lower limb. Ten years after diagnosis, the patient became wheelchaired. Her family history was also known for familial neurodegenerative disease: a 30 year old female sibling with a Friedrich ataxia, and a younger brother with no evidence of disease penetrance, with no family history of sudden cardiac death.
The patient begun her follow up in cardiology department 6 years after diagnosis, since she presented with symptoms of palpitations and atypical chest pain, the annual cardiac checkup included a 12-lead-electrocardiogram that revealed negative T waves in lateral leads, an annual trans thoracic-echocardiography was performed every year for 7 years, and showed no evidence of cardiomyopathy. The patient was prescribed bisoprolol 5 mg per day.
Until this year’s cardiac checkup, when the patient presented to our department with symptoms of palpitations and New York Heart Association (NYHA) class II dyspnea, which she had been experiencing for the last 5 months. She also reported having a general tiredness, and anorexia. However, there was no history of lipothymia or syncope, or other functional signs.
Upon general examination, the patient appeared eupneic at rest with a heart rate of 75 beats per minute, a blood pressure of 122/67 mmHg, a temperature of 36.8°C, room air oxygen saturation was consistently 98%. Cardiovascular examination revealed no abnormal sounds of heart, no signs of heart failure, and no gallops, murmurs or rubs were noticed. Pulmonary auscultation revealed clear breath sounds with no evidence of wheezes, rhonchi, or crackles. Neurological examination had revealed a truncal ataxia, paraparesis of both lower limbs, absent deep tendon reflexes, and dysarthria, while the motor function of both upper limbs is preserved. The rest of general examination was normal.
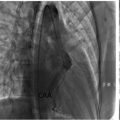
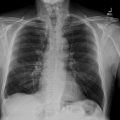
The 12-lead electrocardiogram (EKG) ( Fig. 1 ) revealed regular sinus rhythm at 64 beats per minute, electrical left ventricular hypertrophy (following Cornell index), and presence of negative T waves in lateral and apical leads ( Fig. 1 ). On the 24-hour Holter EKG no ventricular or supraventricular arrhythmias were registered.

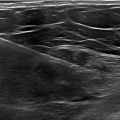
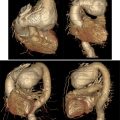
The transthoracic echocardiography was evident for hypertrophic cardiomyopathy with left ventricular (LV) hypertrophy: a maximal wall thickness of 20 mm and a septal wall thickness of 17 mm, a hypertrophic right ventricular wall at 9 mm, and an enlarged inter-atrial septum at 8 mm, with an incomplete anterior systolic movement of the mitral valve, and a left atrial diameter of 31 mm ( Fig. 2 ).

No left ventricular outflow tract (LVOT) obstruction was noted at rest or during exercise (Maximal gradient at 3.35 mmHg at rest and 5 mmHg after Valsalva maneuver), and a maximal gradient post ventricular extrasystole at 10.69 mmHg ( Fig. 1 ). The systolic function of the left ventricle was preserved at 50%. Nevertheless, an alteration of at −11% was noted at the global longitudinal left ventricular strain performed, with regional alteration in anterior and latero-posterior segments as shown in Fig. 3 .