Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer death worldwide and is responsible for 400,000 new cases (4.9% of all cancers) cancer deaths annually. The incidence of EC differs greatly according to geographic region, with the highest incidence in Asian and Middle Eastern countries. In most Western countries, such as the United States, adenocarcinoma has eclipsed squamous cell carcinoma as the predominant histologic type, and it usually afflicts white men. In contrast, squamous cell carcinoma is mostly related to smoking and alcohol consumption in Asia and Middle Eastern countries. Adenocarcinoma is also closely linked with obesity, which, with the associated reflux esophagitis and Barrett preneoplasia, are becoming epidemic in the West and in developed countries.
Historically, the previous standard treatment approach, surgical resection with or without adjuvant therapy, produced cure rates of only about 20%; thus, the addition of preoperative chemoradiation is increasingly being adopted, given the evidence that this approach improves overall survival (OS) relative to surgery alone. The largest of the randomized trials performed in the modern era was a phase III randomized study from a Dutch group, in which 366 evaluable patients were randomized to surgery versus preoperative chemoradiation to 41.4 Gy with carboplatin and paclitaxel. The preoperative chemoradiation substantially improved the median OS time (49.4 months vs. 24.0 months in the surgery-only group). The pathologic complete response rate in the preoperative chemoradiation group was 29% and was higher among patients with squamous cell carcinoma than those with adenocarcinoma (49% vs. 23%; P = .008), which also translated to improved OS from chemoradiation relative to surgery alone for squamous tumors relative to adenocarcinomas (adjusted hazard ratio: 0.42 [95% confidence interval (CI): 0.23–0.79] vs. 0.74 [95% CI: 0.54–1.02]).
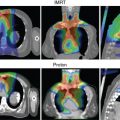
Because of the location of the esophagus, mid- and distal esophageal tumors span posteriorly across the heart and are in very close proximity to the left atrium and anteriorly to the thoracic vertebrae. Proton beam therapy (PBT) is therefore ideal for the treatment of EC, given the tight dose conformality it provides. Comparisons of PBT with three-dimensional (3D) conformal therapy and intensity-modulated (photon) radiation therapy (IMRT) are described in further detail later in this chapter. The following sections describe procedures for treatment simulation, radiation dose and fractionation, target delineation, and treatment verification when using PBT for esophageal tumors, followed by descriptions of aspects of treatment planning that are specific to the two major types of PBT: passive scattering or intensity-modulated proton therapy (IMPT).
Treatment simulation
For treatment simulation, patients with cervical tumors should be supine and immobilized with a five-point mask, with indexed head, neck, and shoulder stabilization. Patients with thoracic or gastroesophageal junction tumors are also positioned supine and immobilized with the use of an indexed upper Vac-Loc/alpha cradle, with both arms up; potential deflation of the cradle must be monitored. The isocenter is placed at the carina. Respiratory motion should be assessed by four-dimensional (4D) scanning, as the esophagus and surrounding structures can move substantially with respiratory motion, particularly at the gastroesophageal junction. Simulation should ideally take place with the patient’s arms above their head to maximize the number of beam arrangements that can be used. To improve reproducibility, patients should be advised to consume nothing by mouth for at least 3 hours before the simulation and before each daily treatment.
Radiation dose and fractionation, target delineation, and treatment verification
A summary of these topics, discussed in further detail below, is provided in Table 17.1 .
| Upper Esophagus Tumors | Lower Esophagus Tumors | |
| GTV (with internal motion) | Gross tumor | Gross tumor |
| CTV | Cervical: superior to cricoid cartilage, inferior 3.5 cm, lateral 1 cm (respecting anatomic boundaries), bilateral SCV fossa Upper thoracic: superior-inferior 3.5 cm, lateral 1 cm (respecting anatomic boundaries) | Middle esophagus: Superior-inferior 3.5 cm, lateral 1 cm (respecting anatomical boundaries), left gastric and celiac lymph nodes not considered unless involved. Distal esophagus/GEJ (Siewert I/II): superior-inferior 3.5 cm, lateral 1 cm (respecting anatomic boundaries), routinely electively cover left gastric lymph nodes, celiac nodes in node-positive disease Siewert type III: treat like gastric cancer |
| Patient setup margin | 0.5–1.0 cm; 0.5 cm if daily kV IGRT is used | 0.5–1.0 cm; 0.5 cm if daily kV IGRT used |
| Prescription dose | 50.4–60 Gy (RBE) in 1.8–2.0 Gy (RBE) fractions | 40–50.4 Gy (RBE) in 1.8–2.0 Gy (RBE) fractions |
Radiation dose and fractionation
Because tumors of the upper esophagus are less likely to be excised surgically, escalation of dose beyond 50.4 Gy can be considered (e.g., 50.4–60 Gy in 1.8- to 2.0-Gy fractions). For tumors of the lower esophagus, the standard dose remains 40 to 50.4 Gy in 1.8- to 2.0-Gy fractions. Dose escalation for lower esophagus tumors can be considered in the context of a clinical trial.
Target delineation
Target delineation differs depending on the location of the tumor within the esophagus. Upper esophagus tumors are defined as those within the cervical and upper thoracic regions, and lower esophagus tumors are in the mid- and distal esophagus, including those at the gastroesophageal junction. Target delineation of Siewert type III gastroesophageal junction tumors should be similar to that of gastric cancers.
For upper esophagus tumors, the gross tumor volume (GTV) encompasses the gross tumor. The clinical target volume (CTV) consists of a 3.5-cm margin superiorly-inferiorly and a 1-cm margin laterally, but not crossing anatomic boundaries (or modified for boundaries such as vessels or bone). However, for cervical esophagus lesions, the upper margin should be the inferior border of the cricoid cartilage. The CTV should also include elective treatment of the supraclavicular fossa bilaterally, even in the absence of involvement.
For lower esophagus tumors, the GTV and CTV are similar to those for upper esophagus tumors, that is, the GTV covers the gross tumor, and the CTV consists of a 3.5-cm margin superiorly-inferiorly and a 1-cm margin laterally, but not crossing anatomic boundaries (or modified for boundaries such as vessels or bone). For lower esophagus tumors, the CTV should also include the left gastric lymph nodes for patients with distal esophagus or gastroesophageal junction tumors (Siewert type I/II disease). For patients with node-positive disease, the celiac axis should also be electively covered, even in the absence of involvement.
For particle therapy, a planning target volume (PTV) is used only for recording and reporting purposes. In practice, the PTV is generated by expanding the CTV with the patient setup margin, which ranges from 0.5 to 1.0 cm based on the image guidance that is available. At MD Anderson, we use daily kV imaging and thus a 0.5-cm PTV margin.
In addition to the PTV, a dosimetric margin is also needed for particle therapy to account for beam range uncertainties and modulations of beams, as described in further detail in the section Treatment Planning.
Treatment verification
For daily treatment verification, patients are positioned as described for treatment simulation, that is, supine and immobilized with either a five-point mask or a Vac-Loc cradle, with the isocenter placed at the carina. Daily kV imaging should be used for all patients. If in-room volumetric imaging (e.g., cone-beam computed tomography [CT] or CT-on-rails) is available, scans can be obtained weekly.
Breathhold and gating techniques are not typically used during the treatment of esophageal tumors, although they can be used for target motion management.
Substantial changes in anatomy or tumor size over the course of treatment are rare, and thus, adaptive simulations are not routinely scheduled. However, if daily or weekly imaging shows changes in normal tissues or tumor, or if the patient undergoes a prolonged treatment break, then we recommend that a verification CT scan be obtained as soon as possible.
Treatment planning
Passive scattering proton beam therapy
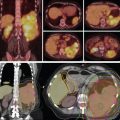
Typically, the beam arrangement for distal tumors is most commonly posteroanterior (PA) and left lateral oblique. However, optimal beam arrangements are determined on a case-by-case basis, and alternative beam arrangements can be used. For proximal to midesophagus tumors, an anteroposterior (AP) and PA beam arrangement could be considered, although caution should be used in the AP direction because beam range uncertainty may lead to irradiation of the spinal cord.
For free-breathing treatments, target coverage during all breathing phases is ensured by creating a planning diaphragm structure from the T0 and T50 phases of the 4D CT scan. The density of the diaphragm is then overridden by using the average Hounsfield units of the maximum-intensity projection scan generated from the 4D CT scans. The treatment plan is then designed with the overridden average CT. This technique ensures adequate coverage to the distal end of the target even with respiratory motion, as shown in Fig. 17.1 A. Aperture design with setup margin and dosimetric margin, beamline design that includes distal and proximal margins based on beam range to account for range uncertainties, and compensator design with smear margin to ensure distal target coverage are illustrated in Fig. 17.1B .